| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:28:49 UTC |
|---|
| Update Date | 2020-06-04 20:40:02 UTC |
|---|
| BMDB ID | BMDB0000335 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 16a-Hydroxyestrone |
|---|
| Description | 16a-Hydroxyestrone, also known as 16a-hydroxyestrone or 16a-hydroxyestrone, belongs to the class of organic compounds known as estrogens and derivatives. These are steroids with a structure containing a 3-hydroxylated estrane. Thus, 16a-hydroxyestrone is considered to be a steroid lipid molecule. 16a-Hydroxyestrone is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. 16a-Hydroxyestrone participates in a number of enzymatic reactions, within cattle. In particular, 16a-Hydroxyestrone can be biosynthesized from estrone; which is catalyzed by the enzyme cytochrome P450 3A5. In addition, 16a-Hydroxyestrone can be converted into estriol through its interaction with the enzyme estradiol 17-beta-dehydrogenase 1. In cattle, 16a-hydroxyestrone is involved in the metabolic pathway called the estrone metabolism pathway. |
|---|
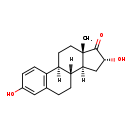
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 16 alpha OHE | ChEBI | | 3,16alpha-Dihydroxy-1,3,5(10)-estratrien-17-one | ChEBI | | 3,16alpha-Dihydroxyestra-1,3,5(10)-trien-17-one | ChEBI | | Estra-1,3,5(10)-triene-3,16alpha-diol-17-one | ChEBI | | 16 a OHE | Generator | | 16 Α ohe | Generator | | 3,16a-Dihydroxy-1,3,5(10)-estratrien-17-one | Generator | | 3,16Α-dihydroxy-1,3,5(10)-estratrien-17-one | Generator | | 3,16a-Dihydroxyestra-1,3,5(10)-trien-17-one | Generator | | 3,16Α-dihydroxyestra-1,3,5(10)-trien-17-one | Generator | | Estra-1,3,5(10)-triene-3,16a-diol-17-one | Generator | | Estra-1,3,5(10)-triene-3,16α-diol-17-one | Generator | | 16 alpha-Hydroxyestrone | HMDB | | 3,16a-Dihydroxy-estra-1,3,5(10)-trien-17-one | HMDB | | 16 beta-Hydroxyestrone | HMDB | | 16-alpha-Hydroxyestrone | HMDB | | 16-Hydroxyestrone | HMDB |
|
|---|
| Chemical Formula | C18H22O3 |
|---|
| Average Molecular Weight | 286.3655 |
|---|
| Monoisotopic Molecular Weight | 286.15689457 |
|---|
| IUPAC Name | (1S,10R,11S,13R,15S)-5,13-dihydroxy-15-methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2(7),3,5-trien-14-one |
|---|
| Traditional Name | (1S,10R,11S,13R,15S)-5,13-dihydroxy-15-methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2(7),3,5-trien-14-one |
|---|
| CAS Registry Number | 566-76-7 |
|---|
| SMILES | [H][C@@]12C[C@@H](O)C(=O)[C@@]1(C)CC[C@]1([H])C3=C(CC[C@@]21[H])C=C(O)C=C3 |
|---|
| InChI Identifier | InChI=1S/C18H22O3/c1-18-7-6-13-12-5-3-11(19)8-10(12)2-4-14(13)15(18)9-16(20)17(18)21/h3,5,8,13-16,19-20H,2,4,6-7,9H2,1H3/t13-,14-,15+,16-,18+/m1/s1 |
|---|
| InChI Key | WPOCIZJTELRQMF-QFXBJFAPSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as estrogens and derivatives. These are steroids with a structure containing a 3-hydroxylated estrane. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Estrane steroids |
|---|
| Direct Parent | Estrogens and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Estrogen-skeleton
- 3-hydroxysteroid
- Hydroxysteroid
- 17-oxosteroid
- Oxosteroid
- 16-alpha-hydroxysteroid
- 16-hydroxysteroid
- Phenanthrene
- Tetralin
- 1-hydroxy-2-unsubstituted benzenoid
- Benzenoid
- Cyclic alcohol
- Secondary alcohol
- Ketone
- Carbonyl group
- Organooxygen compound
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 MEOX; 2 TMS) | splash10-0019-3941100000-3a930f8cc95b733c8653 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 MEOX; 2 TMS) | splash10-0019-3941100000-3e52ec60bd9142c09b74 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-0019-3941100000-3a930f8cc95b733c8653 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-0019-3941100000-3e52ec60bd9142c09b74 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0ab9-2690000000-4da4066a9998d4bb7152 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0gj1-1193300000-a36ac7539d25b407ca9f | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0090000000-ea49b0e7c1a26f8807b3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ap0-0490000000-7a9632ca2f7369b28ad3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gb9-7980000000-f674402195ec376faeb7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-91f32a5e866ea149a4a4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-f8524ff8a5fae66251ad | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02dl-1090000000-5ac386a35fc2bb3cc1f0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0090000000-8269f9806e3a6460adb9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00ks-0590000000-2d8e09d2baef368efec1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dj-0920000000-bce8e0246e58d9e59bbd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-fcaf0435bbf678a207f7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-48bccddc063fe7e2f998 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02di-0090000000-080f7d164203318ad338 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, 100%_DMSO, experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, 100%_DMSO, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|