| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:28:52 UTC |
|---|
| Update Date | 2020-04-22 15:03:02 UTC |
|---|
| BMDB ID | BMDB0000338 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 2-Hydroxyestradiol |
|---|
| Description | 2-Hydroxyestradiol, also known as 2-OH-e2 or ECS, belongs to the class of organic compounds known as estrogens and derivatives. These are steroids with a structure containing a 3-hydroxylated estrane. Thus, 2-hydroxyestradiol is considered to be a steroid. Based on a literature review a significant number of articles have been published on 2-Hydroxyestradiol. |
|---|
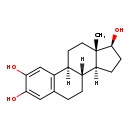
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (17beta)-Estra-1,3,5(10)-triene-2,3,17-triol | ChEBI | | 2-Hydroxyestradiol-17beta | ChEBI | | 2-OH-e2 | ChEBI | | 2-OH-Estradiol | ChEBI | | (17b)-Estra-1,3,5(10)-triene-2,3,17-triol | Generator | | (17Β)-estra-1,3,5(10)-triene-2,3,17-triol | Generator | | 2-Hydroxyestradiol-17b | Generator | | 2-Hydroxyestradiol-17β | Generator | | (17b)-Estra-1,3, 5(10)-triene-2,3,17-triol | HMDB | | (17beta)- Estra-1,3, 5 (10)-triene-2,3,17-triol | HMDB | | 1,3,5(10)-Estratriene-2,3,17Beta-triol | HMDB | | 17beta-2-Hydroxyestradiol | HMDB | | 2,3,17b-Trihydroxyestra-1,3,5(10)-triene | HMDB | | 2-Hydroxy-17beta-estradiol | HMDB | | 2-Hydroxy-estradiol | HMDB | | ECS | HMDB | | Estra-1,3,5 (10)-triene-2,3,17.beta.-triol | HMDB | | Estra-1,3,5(10)-triene-2,3,17-beta-triol | HMDB | | Estra-1,3,5(10)-triene-2,3,17-triol (acd/name 4.0) | HMDB | | Estra-1,3,5(10)-triene-2,3,17b-triol | HMDB | | Estra-1,3,5(10)-triene-2,3,17beta-triol | HMDB | | 2-Hydroxyestradiol-17 alpha | HMDB | | 2-Hydroxyestradiol-17 beta | HMDB | | 2-Hydroxyestradiol, (17alpha)-isomer | HMDB | | 2-Hydroxyestradiol, 4-(14)C-labeled | HMDB |
|
|---|
| Chemical Formula | C18H24O3 |
|---|
| Average Molecular Weight | 288.3814 |
|---|
| Monoisotopic Molecular Weight | 288.172544634 |
|---|
| IUPAC Name | (1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2(7),3,5-triene-4,5,14-triol |
|---|
| Traditional Name | (1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2(7),3,5-triene-4,5,14-triol |
|---|
| CAS Registry Number | 362-05-0 |
|---|
| SMILES | [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])C3=C(CC[C@@]21[H])C=C(O)C(O)=C3 |
|---|
| InChI Identifier | InChI=1S/C18H24O3/c1-18-7-6-11-12(14(18)4-5-17(18)21)3-2-10-8-15(19)16(20)9-13(10)11/h8-9,11-12,14,17,19-21H,2-7H2,1H3/t11-,12+,14-,17-,18-/m0/s1 |
|---|
| InChI Key | DILDHNKDVHLEQB-XSSYPUMDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as estrogens and derivatives. These are steroids with a structure containing a 3-hydroxylated estrane. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Estrane steroids |
|---|
| Direct Parent | Estrogens and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Estrogen-skeleton
- 3-hydroxysteroid
- 2-hydroxysteroid
- Hydroxysteroid
- 17-hydroxysteroid
- Phenanthrene
- Tetralin
- 1-hydroxy-2-unsubstituted benzenoid
- Benzenoid
- Cyclic alcohol
- Secondary alcohol
- Polyol
- Organooxygen compound
- Alcohol
- Hydrocarbon derivative
- Organic oxygen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 190 - 191 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0adr-2490000000-7e8a9e3990faceb5f6d0 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-002u-1002900000-79ac7707883246949511 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-00ei-2950000000-326e2d3fee4fb8b3ca6c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-00di-2910000000-553a7988f4c2019ec5d5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0f96-8975000000-eef1fcf44ed6a27f53a9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dr-0190000000-0e1c17e32d9c1e4063eb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dr-0790000000-01416dcd9eea3747d3c5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-6970000000-d27a7add654c423ec534 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-805faf5743eec20547cb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-c39e06f8fdeeb111e97f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0596-1190000000-36538cb7b1a03d7b2ff7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0090000000-572916cfebc6538c52d0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01w0-0490000000-7a41b9ec75d4c7c28c73 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004j-3910000000-4dd2fe1697c6e685073e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-843a74fabb1d0eb512a1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-40c60966bb1ce4624953 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-1009-0090000000-1c821729dedb3f0fecbe | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, 100%_DMSO, experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, 100%_DMSO, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|