| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:28:58 UTC |
|---|
| Update Date | 2020-04-22 15:03:04 UTC |
|---|
| BMDB ID | BMDB0000344 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 2-Methoxyestradiol-3-methylether |
|---|
| Description | 2-Methoxyestradiol-3-methylether belongs to the class of organic compounds known as estrane steroids. These are steroids with a structure based on the estrane skeleton. Thus, 2-methoxyestradiol-3-methylether is considered to be a steroid. Based on a literature review a significant number of articles have been published on 2-Methoxyestradiol-3-methylether. |
|---|
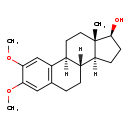
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (17b)-2,3-Dimethoxy-estra-1,3,5(10)-trien-17-ol | HMDB | | 2,3-Dimethoxy-estra-1,3,5(10)-trien-17b-ol | HMDB | | 2-Hydroxy-17b-estradiol 2,3-dimethyl ether | HMDB | | 2-Hydroxyestradiol 2,3-dimethyl ether | HMDB | | 2-Methoxy-17b-estradiol 3-methyl ether | HMDB | | 2-Methoxyestradiol-3-O-methyl ether | HMDB |
|
|---|
| Chemical Formula | C20H28O3 |
|---|
| Average Molecular Weight | 316.4345 |
|---|
| Monoisotopic Molecular Weight | 316.203844762 |
|---|
| IUPAC Name | (1S,10R,11S,14S,15S)-4,5-dimethoxy-15-methyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-2(7),3,5-trien-14-ol |
|---|
| Traditional Name | (1S,10R,11S,14S,15S)-4,5-dimethoxy-15-methyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-2(7),3,5-trien-14-ol |
|---|
| CAS Registry Number | 5976-67-0 |
|---|
| SMILES | [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])C3=C(CC[C@@]21[H])C=C(OC)C(OC)=C3 |
|---|
| InChI Identifier | InChI=1S/C20H28O3/c1-20-9-8-13-14(16(20)6-7-19(20)21)5-4-12-10-17(22-2)18(23-3)11-15(12)13/h10-11,13-14,16,19,21H,4-9H2,1-3H3/t13-,14+,16-,19-,20-/m0/s1 |
|---|
| InChI Key | AVCFXYYRWRZONV-BKRJIHRRSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as estrane steroids. These are steroids with a structure based on the estrane skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Estrane steroids |
|---|
| Direct Parent | Estrane steroids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 17-hydroxysteroid
- Estrane-skeleton
- Hydroxysteroid
- Phenanthrene
- Tetralin
- Anisole
- Alkyl aryl ether
- Benzenoid
- Cyclic alcohol
- Secondary alcohol
- Ether
- Alcohol
- Hydrocarbon derivative
- Organic oxygen compound
- Organooxygen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0f79-1191000000-740c2dcf1c7f466e1fef | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00di-5469000000-bf4c515d54cf43fd866f | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kb-0097000000-ebdac776b10955914131 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014j-0592000000-51bdebfda96665596641 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ll-2390000000-02aeeff3da4ec74dea15 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0029000000-73f255b733327676cfe9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014j-0089000000-261eec7c1edc0d5fc4ab | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05bf-0090000000-a0e5e6c700d1498b8e96 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-d337ec43015bf5d00be6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0019000000-d349dbc3bf02cfbfe31a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01e9-0090000000-59b0f6213d6887844058 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0029000000-dee917e66154967261fc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014m-0494000000-02411aa674b467158464 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a6v-3890000000-696ee37bbc26f1ce2daf | View in MoNA |
|---|
|
|---|
| Synthesis Reference | Rao, P. Narashimha; Axelrod, Leonard R. 2-Hydroxyestrogens. II. Synthesis of 2,3-dihydroxyestra-1,3,5(10)-trien-17-one and estra-1,3,5(10)-triene-2,3,16a,17b-tetraol. Journal of the Chemical Society (1961), 4769-73. |
|---|