| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:31:28 UTC |
|---|
| Update Date | 2020-04-22 15:03:49 UTC |
|---|
| BMDB ID | BMDB0000488 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 4E,15Z-Bilirubin IXa |
|---|
| Description | Bilirubin belongs to the class of organic compounds known as bilirubins. These are organic compounds containing a dicarboxylic acyclic tetrapyrrole derivative. Bilirubin is possibly soluble (in water) and an extremely weak basic (essentially neutral) compound (based on its pKa). Bilirubin exists in all living species, ranging from bacteria to humans. In cattle, bilirubin is involved in the metabolic pathway called the porphyrin metabolism pathway. Bilirubin is a potentially toxic compound. |
|---|
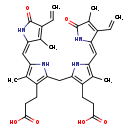
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (4E)2,17-Diethenyl-1,10,19,22,23,24-hexahydro-3,7,13,18-tetramethyl-1,19-dioxo--21H-biline-8,12-dipropanoic acid | ChEBI | | (4E)2,17-Diethenyl-1,10,19,22,23,24-hexahydro-3,7,13,18-tetramethyl-1,19-dioxo--21H-biline-8,12-dipropanoate | Generator | | (4E,15Z)-Bilirubin ixa | HMDB | | (4E,15Z)-Bilirubin | HMDB | | (4E,15Z)-Bilirubin IXalpha | HMDB | | (4E,15Z)-Bilirubin IXα | HMDB | | 4E,15Z-Bilirubin IXa | HMDB | | 4E,15Z-Bilirubin IXalpha | HMDB | | 4E,15Z-Bilirubin IXα | HMDB |
|
|---|
| Chemical Formula | C33H36N4O6 |
|---|
| Average Molecular Weight | 584.6621 |
|---|
| Monoisotopic Molecular Weight | 584.263484904 |
|---|
| IUPAC Name | 3-(2-{[3-(2-carboxyethyl)-5-{[(2E)-4-ethenyl-3-methyl-5-oxo-2,5-dihydro-1H-pyrrol-2-ylidene]methyl}-4-methyl-1H-pyrrol-2-yl]methyl}-5-{[(2Z)-3-ethenyl-4-methyl-5-oxo-2,5-dihydro-1H-pyrrol-2-ylidene]methyl}-4-methyl-1H-pyrrol-3-yl)propanoic acid |
|---|
| Traditional Name | 3-(2-{[3-(2-carboxyethyl)-5-{[(2E)-4-ethenyl-3-methyl-5-oxo-1H-pyrrol-2-ylidene]methyl}-4-methyl-1H-pyrrol-2-yl]methyl}-5-{[(2Z)-3-ethenyl-4-methyl-5-oxo-1H-pyrrol-2-ylidene]methyl}-4-methyl-1H-pyrrol-3-yl)propanoic acid |
|---|
| CAS Registry Number | 635-65-4 |
|---|
| SMILES | CC1=C(C=C)\C(NC1=O)=C\C1=C(C)C(CCC(O)=O)=C(CC2=C(CCC(O)=O)C(C)=C(N2)\C=C2\NC(=O)C(C=C)=C2C)N1 |
|---|
| InChI Identifier | InChI=1S/C33H36N4O6/c1-7-20-19(6)32(42)37-27(20)14-25-18(5)23(10-12-31(40)41)29(35-25)15-28-22(9-11-30(38)39)17(4)24(34-28)13-26-16(3)21(8-2)33(43)36-26/h7-8,13-14,34-35H,1-2,9-12,15H2,3-6H3,(H,36,43)(H,37,42)(H,38,39)(H,40,41)/b26-13+,27-14- |
|---|
| InChI Key | BPYKTIZUTYGOLE-KDUUSRDASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as bilirubins. These are organic compounds containing a dicarboxylic acyclic tetrapyrrole derivative. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrapyrroles and derivatives |
|---|
| Sub Class | Bilirubins |
|---|
| Direct Parent | Bilirubins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Bilirubin skeleton
- Dicarboxylic acid or derivatives
- Substituted pyrrole
- Pyrrole
- Pyrroline
- Heteroaromatic compound
- Secondary carboxylic acid amide
- Lactam
- Carboxamide group
- Azacycle
- Carboxylic acid
- Carboxylic acid derivative
- Organopnictogen compound
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("(4E,15Z)-Bilirubin,1TMS,#1" TMS) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_9) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_10) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_11) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_12) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_13) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_14) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_15) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-IT , negative | splash10-000i-0090000000-50ff729317f07f6120b5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0000090000-651b4bf16c771e7c6f66 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01dr-0110290000-4279e28059ff64b01830 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0hox-3103930000-84aed34ed98f48f2e50f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00lr-0000090000-3e6982456f8db869f1f9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-067r-1010190000-16aaef818cf422a2b166 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9010230000-4f5bb6a71d8961e42485 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-0010090000-d21be2c11a0dfd17c6e9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kr-0030190000-749f82b203e182b3e46f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0kac-0291230000-962a583eff6dd8d5cf73 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0010090000-467141ca1354a02b0aef | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00s9-0030390000-ecd6322d5a093ef12c52 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000f-0090400000-57eee3679925f2fa12ee | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, 100%_DMSO, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, C, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, DMSO, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, DMSO, experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, 100%_DMSO, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|