| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:31:47 UTC |
|---|
| Update Date | 2020-05-11 20:28:02 UTC |
|---|
| BMDB ID | BMDB0000503 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
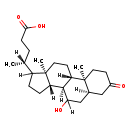
| Common Name | 7a-Hydroxy-3-oxo-5b-cholanoic acid |
|---|
| Description | 7a-Hydroxy-3-oxo-5b-cholanoic acid is an extremely weak basic (essentially neutral) compound (based on its pKa). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 7a-Hydroxy-3-oxo-5b-cholanoate | Generator | | (4R)-4-[(1S,2S,7R,10R,11S,15R)-9-Hydroxy-2,15-dimethyl-5-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-yl]pentanoate | Generator | | (4R)-4-[(1S,2S,7R,10R,11S,15R)-9-Hydroxy-2,15-dimethyl-5-oxotetracyclo[8.7.0.0,.0,]heptadecan-14-yl]pentanoate | Generator |
|
|---|
| Chemical Formula | C24H38O4 |
|---|
| Average Molecular Weight | 390.5561 |
|---|
| Monoisotopic Molecular Weight | 390.277009704 |
|---|
| IUPAC Name | (4R)-4-[(1S,2S,7R,10R,11S,15R)-9-hydroxy-2,15-dimethyl-5-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-yl]pentanoic acid |
|---|
| Traditional Name | (4R)-4-[(1S,2S,7R,10R,11S,15R)-9-hydroxy-2,15-dimethyl-5-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-yl]pentanoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@@](C)(CCC(O)=O)C1([H])CC[C@@]2([H])[C@]3([H])C([H])(O)C[C@]4([H])CC(=O)CC[C@]4(C)[C@@]3([H])CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C24H38O4/c1-14(4-7-21(27)28)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(25)12-15(23)13-20(22)26/h14-15,17-20,22,26H,4-13H2,1-3H3,(H,27,28)/t14-,15+,17?,18+,19+,20?,22+,23+,24-/m1/s1 |
|---|
| InChI Key | KNVADAPHVNKTEP-VWEUZOFESA-N |
|---|
| Chemical Taxonomy |
|---|
| Classification | Not classified |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0006-0009000000-c2f02514f67b223e21af | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0007-0907000000-f9b64f6e2d553d1468c2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0007-0809000000-ca5457440e9077b8c9a1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05fr-0009000000-fafd5a671c61f81c15c8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-076s-0009000000-70b658ddea6d96ced853 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01t9-1319000000-ef02c40883611a4c3308 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-bcd0337fb08276e5242b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00dr-0009000000-fb7ca64898cc60921257 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9006000000-c936cb19bcd9e5afce3c | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, 100%_DMSO, experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, 100%_DMSO, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|
| Synthesis Reference | Riva, Sergio; Bovara, Roberto; Zetta, Lucia; Pasta, Piero; Ottolina, Gianluca; Carrea, Giacomo. Enzymic a/b inversion of C-3 hydroxyl of bile acids and study of the effects of organic solvents on reaction rates. Journal of Organic Chemistry (1988), 53(1), 88-92. |
|---|