| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:34:53 UTC |
|---|
| Update Date | 2020-05-11 20:21:20 UTC |
|---|
| BMDB ID | BMDB0000686 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
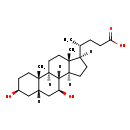
| Common Name | Isoursodeoxycholic acid |
|---|
| Description | Isoursodeoxycholic acid, also known as iso-ursodeoxycholate, belongs to the class of organic compounds known as dihydroxy bile acids, alcohols and derivatives. Dihydroxy bile acids, alcohols and derivatives are compounds containing or derived from a bile acid or alcohol, and which bears exactly two carboxylic acid groups. Based on a literature review a small amount of articles have been published on Isoursodeoxycholic acid. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3beta,5beta,7beta)-3,7-Dihydroxycholan-24-Oic acid | ChEBI | | 3beta,7beta-Dihydroxy-5beta-cholan-24-Oic acid | ChEBI | | 3beta,7beta-Dihydroxy-5beta-cholanoic acid | ChEBI | | ISO-ursodeoxycholIC ACID | ChEBI | | (3b,5b,7b)-3,7-Dihydroxycholan-24-Oate | Generator | | (3b,5b,7b)-3,7-Dihydroxycholan-24-Oic acid | Generator | | (3beta,5beta,7beta)-3,7-Dihydroxycholan-24-Oate | Generator | | (3Β,5β,7β)-3,7-dihydroxycholan-24-Oate | Generator | | (3Β,5β,7β)-3,7-dihydroxycholan-24-Oic acid | Generator | | 3b,7b-Dihydroxy-5b-cholan-24-Oate | Generator | | 3b,7b-Dihydroxy-5b-cholan-24-Oic acid | Generator | | 3beta,7beta-Dihydroxy-5beta-cholan-24-Oate | Generator | | 3Β,7β-dihydroxy-5β-cholan-24-Oate | Generator | | 3Β,7β-dihydroxy-5β-cholan-24-Oic acid | Generator | | 3b,7b-Dihydroxy-5b-cholanoate | Generator | | 3b,7b-Dihydroxy-5b-cholanoic acid | Generator | | 3beta,7beta-Dihydroxy-5beta-cholanoate | Generator | | 3Β,7β-dihydroxy-5β-cholanoate | Generator | | 3Β,7β-dihydroxy-5β-cholanoic acid | Generator | | ISO-ursodeoxycholate | Generator | | Isoursodeoxycholate | Generator | | 3b,7b-Dihydroxy-5b-cholanate | HMDB | | 3b,7b-Dihydroxy-5b-cholanic acid | HMDB | | 3b-Ursodeoxycholate | HMDB | | 3b-Ursodeoxycholic acid | HMDB | | 3,7-Dihydroxycholan-24-Oic acid | MeSH, HMDB | | Sodium OF isoursodeoxycholic acid | MeSH, HMDB | | 3beta,7beta-Dihydroxy-5beta-cholanic acid | HMDB | | 3beta-Ursodeoxycholic acid | HMDB | | 3β,7β-Dihydroxy-5β-cholanic acid | HMDB | | 3β-Ursodeoxycholic acid | HMDB | | Isoursodeoxycholic acid | HMDB |
|
|---|
| Chemical Formula | C24H40O4 |
|---|
| Average Molecular Weight | 392.572 |
|---|
| Monoisotopic Molecular Weight | 392.292659768 |
|---|
| IUPAC Name | (4R)-4-[(1S,2S,5S,7S,9S,10R,11S,14R,15R)-5,9-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-yl]pentanoic acid |
|---|
| Traditional Name | (4R)-4-[(1S,2S,5S,7S,9S,10R,11S,14R,15R)-5,9-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-yl]pentanoic acid |
|---|
| CAS Registry Number | 78919-26-3 |
|---|
| SMILES | [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C24H40O4/c1-14(4-7-21(27)28)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(25)12-15(23)13-20(22)26/h14-20,22,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15+,16+,17-,18+,19+,20+,22+,23+,24-/m1/s1 |
|---|
| InChI Key | RUDATBOHQWOJDD-DNMBCGTGSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dihydroxy bile acids, alcohols and derivatives. Dihydroxy bile acids, alcohols and derivatives are compounds containing or derived from a bile acid or alcohol, and which bears exactly two carboxylic acid groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
| Direct Parent | Dihydroxy bile acids, alcohols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dihydroxy bile acid, alcohol, or derivatives
- 3-hydroxysteroid
- 7-hydroxysteroid
- 7-alpha-hydroxysteroid
- 3-beta-hydroxysteroid
- Hydroxysteroid
- Cyclic alcohol
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic oxide
- Alcohol
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Organooxygen compound
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | - dihydroxy-5beta-cholanic acid (CHEBI:43419 )
- C24 bile acids, alcohols, and derivatives (C17662 )
- C24 bile acids, alcohols, and derivatives (LMST04010035 )
|
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01rt-0529000000-3b28c649fc51c7806ad3 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0006-1110390000-52bc66ab11fd14425d4f | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-056r-0009000000-997e61e986e67265241c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-056r-0009000000-81134947694f847d1c65 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02t9-1219000000-6a0ddebebacac090c27e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0009000000-efdaad69eee0ae934dd5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-006x-1009000000-15b4edab9d05e4c3693a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9006000000-9291e69db3a3c47ecd97 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0009000000-7631731446db77938069 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-006x-0009000000-39e7e555dc6b36ada2af | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-1009000000-351c21e3dab693818b22 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0019000000-56dc67f71a8507c1433f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-3039000000-510702cae19e3906b6e1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9550000000-7486a5f56afd302efa7e | View in MoNA |
|---|
|
|---|