| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:35:30 UTC |
|---|
| Update Date | 2020-05-11 20:09:09 UTC |
|---|
| BMDB ID | BMDB0000722 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Lithocholyltaurine |
|---|
| Description | Lithocholyltaurine, also known as taurolithocholate, belongs to the class of organic compounds known as taurinated bile acids and derivatives. These are bile acid derivatives containing a taurine conjugated to the bile acid moiety. Lithocholyltaurine is an extremely weak basic (essentially neutral) compound (based on its pKa). |
|---|
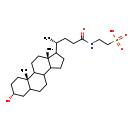
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-[(3alpha-Hydroxy-24-oxo-5beta-cholan-24-yl)amino]ethanesulfonic acid | HMDB | | 2-[[(3a,5b)-3-Hydroxy-24-oxocholan-24-yl]amino]-ethanesulfonate | HMDB | | 2-[[(3a,5b)-3-Hydroxy-24-oxocholan-24-yl]amino]-ethanesulfonic acid | HMDB | | 3a-Hydroxy-5b-cholanoyltaurine | HMDB | | 3a-Hydroxy-N-(2-sulfoethyl)-5b-cholan-24-amide | HMDB | | Cholane ethanesulfonic acid deriv. | HMDB | | Lithocholic acid taurine conjugate | HMDB | | Lithocholic acid taurine conjugic acid | HMDB | | N-(3a-Hydroxy-5b-cholan-24-oyl)-taurine | HMDB | | Taurolithocholate | HMDB | | Taurolithocholic acid | HMDB | | Lithocholate, taurine | HMDB | | Taurine lithocholate | HMDB | | Taurolithocholic acid, monosodium salt | HMDB | | Acid, taurolithocholic | HMDB |
|
|---|
| Chemical Formula | C26H45NO5S |
|---|
| Average Molecular Weight | 483.704 |
|---|
| Monoisotopic Molecular Weight | 483.301844245 |
|---|
| IUPAC Name | 2-[(4R)-4-[(2S,5R,15R)-5-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-yl]pentanamido]ethane-1-sulfonic acid |
|---|
| Traditional Name | 2-[(4R)-4-[(2S,5R,15R)-5-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-yl]pentanamido]ethanesulfonic acid |
|---|
| CAS Registry Number | 516-90-5 |
|---|
| SMILES | C[C@H](CCC(=O)NCCS(O)(=O)=O)C1CCC2C3CCC4C[C@H](O)CC[C@]4(C)C3CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C26H45NO5S/c1-17(4-9-24(29)27-14-15-33(30,31)32)21-7-8-22-20-6-5-18-16-19(28)10-12-25(18,2)23(20)11-13-26(21,22)3/h17-23,28H,4-16H2,1-3H3,(H,27,29)(H,30,31,32)/t17-,18?,19-,20?,21?,22?,23?,25+,26-/m1/s1 |
|---|
| InChI Key | QBYUNVOYXHFVKC-LVMSMGIASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as taurinated bile acids and derivatives. These are bile acid derivatives containing a taurine conjugated to the bile acid moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
| Direct Parent | Taurinated bile acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Taurinated bile acid
- Hydroxy bile acid, alcohol, or derivatives
- Monohydroxy bile acid, alcohol, or derivatives
- 3-hydroxysteroid
- Hydroxysteroid
- 3-alpha-hydroxysteroid
- Fatty amide
- N-acyl-amine
- Fatty acyl
- Sulfonyl
- Organosulfonic acid
- Organosulfonic acid or derivatives
- Organic sulfonic acid or derivatives
- Alkanesulfonic acid
- Cyclic alcohol
- Secondary carboxylic acid amide
- Secondary alcohol
- Carboxamide group
- Carboxylic acid derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organonitrogen compound
- Organosulfur compound
- Organooxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 212 - 213 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | |
|---|
| Synthesis Reference | Zhang, Jie; Griffiths, William J.; Bergman, Tomas; Sjoevall, Jan. Derivatization of bile acids with taurine for analysis by fast atom bombardment mass spectrometry with collision-induced fragmentation. Journal of Lipid Research (1993), 34(11), 1895-900. |
|---|