| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:35:54 UTC |
|---|
| Update Date | 2020-04-22 15:05:07 UTC |
|---|
| BMDB ID | BMDB0000746 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Hydroxyisocaproic acid |
|---|
| Description | Hydroxyisocaproic acid, also known as (S)-2-hydroxyisocaproate or (S)-leucic acid, belongs to the class of organic compounds known as hydroxy fatty acids. These are fatty acids in which the chain bears a hydroxyl group. Based on a literature review a significant number of articles have been published on Hydroxyisocaproic acid. |
|---|
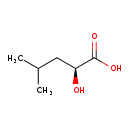
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+)-2-Hydroxyisocaproic acid | ChEBI | | (+)-alpha-Hydroxyisocaproic acid | ChEBI | | (S)-2-Hydroxyisocaproic acid | ChEBI | | (S)-Leucic acid | ChEBI | | 2-HYDROXY-4-methyl-pentanoIC ACID | ChEBI | | L-2-Hydroxy-4-methylvaleric acid | ChEBI | | L-2-Hydroxyisocaproic acid | ChEBI | | L-alpha-Hydroxyisocaproic acid | ChEBI | | L-Leucic acid | ChEBI | | (+)-2-Hydroxyisocaproate | Generator | | (+)-a-Hydroxyisocaproate | Generator | | (+)-a-Hydroxyisocaproic acid | Generator | | (+)-alpha-Hydroxyisocaproate | Generator | | (+)-Α-hydroxyisocaproate | Generator | | (+)-Α-hydroxyisocaproic acid | Generator | | (S)-2-Hydroxyisocaproate | Generator | | (S)-Leucate | Generator | | 2-HYDROXY-4-methyl-pentanoate | Generator | | L-2-Hydroxy-4-methylvalerate | Generator | | L-2-Hydroxyisocaproate | Generator | | L-a-Hydroxyisocaproate | Generator | | L-a-Hydroxyisocaproic acid | Generator | | L-alpha-Hydroxyisocaproate | Generator | | L-Α-hydroxyisocaproate | Generator | | L-Α-hydroxyisocaproic acid | Generator | | L-Leucate | Generator | | Hydroxyisocaproate | Generator | | Hydroxy-isocaproate | HMDB | | (2S)-2-Hydroxy-4-methylpentanoate | HMDB | | (2S)-2-Hydroxy-4-methylpentanoic acid | HMDB | | (S)-2-Hydroxy-4-methyl-pentanoate | HMDB | | (S)-2-Hydroxy-4-methyl-pentanoic acid | HMDB | | S-2-Hydroxy-4-methylpentanoate | HMDB | | S-2-Hydroxy-4-methylpentanoic acid | HMDB |
|
|---|
| Chemical Formula | C6H12O3 |
|---|
| Average Molecular Weight | 132.1577 |
|---|
| Monoisotopic Molecular Weight | 132.07864425 |
|---|
| IUPAC Name | (2S)-2-hydroxy-4-methylpentanoic acid |
|---|
| Traditional Name | (+)-α-hydroxyisocaproate |
|---|
| CAS Registry Number | 13748-90-8 |
|---|
| SMILES | CC(C)C[C@H](O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C6H12O3/c1-4(2)3-5(7)6(8)9/h4-5,7H,3H2,1-2H3,(H,8,9)/t5-/m0/s1 |
|---|
| InChI Key | LVRFTAZAXQPQHI-YFKPBYRVSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hydroxy fatty acids. These are fatty acids in which the chain bears a hydroxyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
| Direct Parent | Hydroxy fatty acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Branched fatty acid
- Hydroxy fatty acid
- Methyl-branched fatty acid
- Alpha-hydroxy acid
- Hydroxy acid
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Adiposome
- Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 78 - 80 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9000000000-866999574fff80fabad1 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-02gc-9440000000-7fffd162011678b17f4a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Negative (Annotated) | splash10-001r-7900000000-716c5f55d60f6894323b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Negative (Annotated) | splash10-014s-9000000000-9c50133cdf095ca16a2e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Negative (Annotated) | splash10-00kb-9100000000-dc2c3e9343c9a8e307c3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0159-5900000000-01e9c211c49cea6fc5c6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ap0-9300000000-a1f6fc289aa530d63fd8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-eb5a5d102c8e756f6834 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-2900000000-1a66b9767516fbd45630 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-06s9-9400000000-cf71f1c20e2a87bbb2b0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000000-11e049c8df5b1a6268c9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0019-9700000000-a7d0b1ce1c211b31bc0d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00kr-9100000000-504826b66e199a65ef09 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014l-9000000000-ccecb4a470c359949fec | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-9000000000-3019a9d0620e7da01963 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kf-9000000000-e587d44396e53f2409fa | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-457ece1d510dad645c89 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 90 MHz, CDCl3, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 25.16 MHz, CDCl3, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|