| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:35:58 UTC |
|---|
| Update Date | 2020-05-05 18:37:19 UTC |
|---|
| BMDB ID | BMDB0000749 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
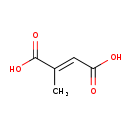
| Common Name | Mesaconic acid |
|---|
| Description | Citraconic acid, also known as 2-methylmaleate or methylmaleic acid, belongs to the class of organic compounds known as methyl-branched fatty acids. These are fatty acids with an acyl chain that has a methyl branch. Usually, they are saturated and contain only one or more methyl group. However, branches other than methyl may be present. Citraconic acid exists as a solid, very hydrophobic, practically insoluble (in water), and relatively neutral molecule. Citraconic acid exists in all living organisms, ranging from bacteria to humans. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (e)-2-Methyl-2-butenedioic acid | ChEBI | | (e)-Citraconic acid | ChEBI | | 2-Methylfumaric acid | ChEBI | | Citronic acid | ChEBI | | Methylfumaric acid | ChEBI | | trans-1-Propene-1,2-dicarboxylic acid | ChEBI | | trans-2-Methyl-2-butenedioic acid | ChEBI | | 2-Methylfumarate | Kegg | | (e)-2-Methyl-2-butenedioate | Generator | | (e)-Citraconate | Generator | | Citronate | Generator | | Methylfumarate | Generator | | trans-1-Propene-1,2-dicarboxylate | Generator | | trans-2-Methyl-2-butenedioate | Generator | | Mesaconate | Generator | | (Z)-2-Methyl-2-butenedioic acid | HMDB | | Citraconic acid | HMDB | | Citraconic acid, (e)-isomer | HMDB | | Citraconic acid, ammonium salt | HMDB | | Citraconic acid, calcium salt | HMDB | | Citraconic acid, sodium salt | HMDB | | Methylmaleic acid | HMDB | | Monomethylfumarate | HMDB | | (2E)-2-Methyl-2-butenedioate | HMDB | | (2E)-2-Methyl-2-butenedioic acid | HMDB |
|
|---|
| Chemical Formula | C5H6O4 |
|---|
| Average Molecular Weight | 130.0987 |
|---|
| Monoisotopic Molecular Weight | 130.02660868 |
|---|
| IUPAC Name | (2E)-2-methylbut-2-enedioic acid |
|---|
| Traditional Name | mesaconic acid |
|---|
| CAS Registry Number | 498-24-8 |
|---|
| SMILES | C\C(=C/C(O)=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C5H6O4/c1-3(5(8)9)2-4(6)7/h2H,1H3,(H,6,7)(H,8,9)/b3-2+ |
|---|
| InChI Key | HNEGQIOMVPPMNR-NSCUHMNNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as methyl-branched fatty acids. These are fatty acids with an acyl chain that has a methyl branch. Usually, they are saturated and contain only one or more methyl group. However, branches other than methyl may be present. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
| Direct Parent | Methyl-branched fatty acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Methyl-branched fatty acid
- Unsaturated fatty acid
- Dicarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Adiposome
- Cell membrane
- Cytoplasm
- Membrane
- Mitochondria
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 200 - 202 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 26.3 mg/mL at 18 °C | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (2 TMS) | splash10-0002-1910000000-7b5cb889d8459c0edd01 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (2 TMS) | splash10-00di-9500000000-b21cd4d2b489173d2933 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (2 TMS) | splash10-053r-2940000000-4329a46b213ac4abcca9 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0002-1910000000-7b5cb889d8459c0edd01 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00di-9500000000-b21cd4d2b489173d2933 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-053r-2940000000-4329a46b213ac4abcca9 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01q3-9200000000-99f5b89fc0026d764b2b | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-05g3-9540000000-ba938fe63ed9356955c6 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-000j-9400000000-e10edb30d7748a7adb05 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-00m0-9000000000-6ef60fd2abfbb5741bca | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0159-9000000000-e14ffb74e5a8298232a3 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-000i-9100000000-8749d95b95760456aeed | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-000i-9100000000-8749d95b95760456aeed | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-5900000000-48835a534a81752d3085 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kr-9100000000-dcbf0f65cbb6f9b6b578 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-9000000000-632723c704c4db90a4b6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004r-5900000000-4b2260c72d18030ec912 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002r-9500000000-1fd59395c864782b12b9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014u-9000000000-b6d65140f365dabf3b3a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01p9-9400000000-ebf4634a042628bb8a44 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00ko-9000000000-e04449c635203c9d3da0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-1b2c407328e3780e996e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-9000000000-ec20127c74818b1f634d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-9000000000-8867d7b163c801abd185 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014l-9000000000-55fcd5974652df621054 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|