| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:36:19 UTC |
|---|
| Update Date | 2020-05-11 20:21:36 UTC |
|---|
| BMDB ID | BMDB0000768 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | N-Acetyl-9-O-lactoylneuraminic acid |
|---|
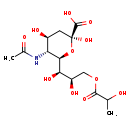
| Description | N-Acetyl-9-O-lactoylneuraminic acid, also known as 9-O-lactoyl-N-acetylneuraminic acid or neu5,8ac29, belongs to the class of organic compounds known as n-acylneuraminic acids. These are neuraminic acids carrying an N-acyl substituent. Based on a literature review a small amount of articles have been published on N-Acetyl-9-O-lactoylneuraminic acid. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| N-Acetyl-9-O-lactoylneuraminate | Generator | | 5-(Acetylamino)-3,5-dideoxy-9-(2-hydroxypropanoate | HMDB | | 5-(Acetylamino)-3,5-dideoxy-9-(2-hydroxypropanoate) D-glycero-D-galacto-2-nonulosonate | HMDB | | 5-(Acetylamino)-3,5-dideoxy-9-(2-hydroxypropanoate) D-glycero-D-galacto-2-nonulosonic acid | HMDB | | 5-(Acetylamino)-3,5-dideoxy-9-(2-hydroxypropanoic acid | HMDB | | 9-O-Lactoyl-N-acetylneuraminic acid | HMDB | | Neu5,8ac29 | HMDB | | Neu5ac9LT | HMDB | | (2S,4S,5R,6R)-6-[(1R,2R)-1,2-Dihydroxy-3-[(2-hydroxypropanoyl)oxy]propyl]-2,4-dihydroxy-5-[(1-hydroxyethylidene)amino]oxane-2-carboxylate | HMDB | | N-Acetyl-9-O-lactoylneuraminic acid | MeSH |
|
|---|
| Chemical Formula | C14H23NO11 |
|---|
| Average Molecular Weight | 381.3325 |
|---|
| Monoisotopic Molecular Weight | 381.127110583 |
|---|
| IUPAC Name | (2S,4S,5R,6R)-6-[(1R,2R)-1,2-dihydroxy-3-[(2-hydroxypropanoyl)oxy]propyl]-5-acetamido-2,4-dihydroxyoxane-2-carboxylic acid |
|---|
| Traditional Name | (2S,4S,5R,6R)-6-[(1R,2R)-1,2-dihydroxy-3-[(2-hydroxypropanoyl)oxy]propyl]-5-acetamido-2,4-dihydroxyoxane-2-carboxylic acid |
|---|
| CAS Registry Number | 92935-30-3 |
|---|
| SMILES | CC(O)C(=O)OC[C@@H](O)[C@@H](O)[C@@H]1O[C@@](O)(C[C@H](O)[C@H]1NC(C)=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C14H23NO11/c1-5(16)12(21)25-4-8(19)10(20)11-9(15-6(2)17)7(18)3-14(24,26-11)13(22)23/h5,7-11,16,18-20,24H,3-4H2,1-2H3,(H,15,17)(H,22,23)/t5?,7-,8+,9+,10+,11+,14-/m0/s1 |
|---|
| InChI Key | XXNWSGSWDRDYLR-NVZZXFSUSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acylneuraminic acids. These are neuraminic acids carrying an N-acyl substituent. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | N-acylneuraminic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-acylneuraminic acid
- Neuraminic acid
- C-glucuronide
- C-glycosyl compound
- Glycosyl compound
- Alpha-hydroxy acid
- Dicarboxylic acid or derivatives
- Pyran
- Oxane
- Hydroxy acid
- Acetamide
- Carboxamide group
- Carboxylic acid ester
- Secondary carboxylic acid amide
- Secondary alcohol
- Hemiacetal
- Carboxylic acid derivative
- Oxacycle
- Carboxylic acid
- Organoheterocyclic compound
- Alcohol
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Organic nitrogen compound
- Organopnictogen compound
- Organonitrogen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01ow-8595000000-22490b282838f0620ce4 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-014i-1900312000-dce815b71d4f334c4c6f | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_4_29) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("N-Acetyl-9-O-lactoylneuraminic acid,4TBDMS,#29" TMS) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_9) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_10) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_11) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_12) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03kc-2039000000-ffda67dcdbf5989adc05 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-5597000000-b3026a03337e85591b9b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03kd-9231000000-7f1bdaa0dc29dabd774c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01w1-8694000000-1f16ceda4dac4e54ea7f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0079-9220000000-20a0c48e8b43be8736a1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0079-9300000000-0ac21507b6af44e18181 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0019000000-974bbe54e08018525402 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0uki-4091000000-4c1a7df73bb0ef83f182 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006x-9120000000-d82807fbd427bbd40342 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03ec-0059000000-c329c6816a441438b833 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-02tc-1094000000-a1a3c6f2d948b33c321a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0007-6970000000-a21ee52cc71df25cfe45 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|