| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:36:27 UTC |
|---|
| Update Date | 2020-04-22 15:05:16 UTC |
|---|
| BMDB ID | BMDB0000781 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
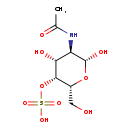
| Common Name | N-Acetylgalactosamine 4-sulphate |
|---|
| Description | N-Acetylgalactosamine 4-sulphate, also known as beta-D-galpnac4S or GALNAC4S, belongs to the class of organic compounds known as acylaminosugars. These are organic compounds containing a sugar linked to a chain through N-acyl group. N-Acetylgalactosamine 4-sulphate is an extremely weak basic (essentially neutral) compound (based on its pKa). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| N-Acetyl-beta-D-galactosamine 4-sulphate | ChEBI | | 2-(Acetylamino)-2-deoxy-4-O-sulfO-beta-D-galactopyranose | ChEBI | | 2-Deoxy-2-acetamido-beta-D-galactose-4-sulfate | ChEBI | | beta-D-GalpNAc4S | ChEBI | | N-Acetyl-beta-D-galactosamine 4-sulfuric acid | ChEBI | | N-Acetyl-b-D-galactosamine 4-sulfate | Generator | | N-Acetyl-b-D-galactosamine 4-sulfuric acid | Generator | | N-Acetyl-b-D-galactosamine 4-sulphate | Generator | | N-Acetyl-b-D-galactosamine 4-sulphuric acid | Generator | | N-Acetyl-beta-D-galactosamine 4-sulfate | Generator | | N-Acetyl-beta-D-galactosamine 4-sulphuric acid | Generator | | N-Acetyl-β-D-galactosamine 4-sulfate | Generator | | N-Acetyl-β-D-galactosamine 4-sulfuric acid | Generator | | N-Acetyl-β-D-galactosamine 4-sulphate | Generator | | N-Acetyl-β-D-galactosamine 4-sulphuric acid | Generator | | 2-(Acetylamino)-2-deoxy-4-O-sulfO-b-D-galactopyranose | Generator | | 2-(Acetylamino)-2-deoxy-4-O-sulfO-β-D-galactopyranose | Generator | | 2-(Acetylamino)-2-deoxy-4-O-sulphO-b-D-galactopyranose | Generator | | 2-(Acetylamino)-2-deoxy-4-O-sulphO-beta-D-galactopyranose | Generator | | 2-(Acetylamino)-2-deoxy-4-O-sulphO-β-D-galactopyranose | Generator | | 2-Deoxy-2-acetamido-b-D-galactose-4-sulfate | Generator | | 2-Deoxy-2-acetamido-b-D-galactose-4-sulfuric acid | Generator | | 2-Deoxy-2-acetamido-b-D-galactose-4-sulphate | Generator | | 2-Deoxy-2-acetamido-b-D-galactose-4-sulphuric acid | Generator | | 2-Deoxy-2-acetamido-beta-D-galactose-4-sulfuric acid | Generator | | 2-Deoxy-2-acetamido-beta-D-galactose-4-sulphate | Generator | | 2-Deoxy-2-acetamido-beta-D-galactose-4-sulphuric acid | Generator | | 2-Deoxy-2-acetamido-β-D-galactose-4-sulfate | Generator | | 2-Deoxy-2-acetamido-β-D-galactose-4-sulfuric acid | Generator | | 2-Deoxy-2-acetamido-β-D-galactose-4-sulphate | Generator | | 2-Deoxy-2-acetamido-β-D-galactose-4-sulphuric acid | Generator | | b-D-GalpNAc4S | Generator | | Β-D-galpnac4S | Generator | | N-Acetylgalactosamine 4-sulfate | Generator | | N-Acetylgalactosamine 4-sulfuric acid | Generator | | N-Acetylgalactosamine 4-sulphuric acid | Generator | | 2-(Acetylamino)-2-deoxy-D-galactose 4-(hydrogen sulfate) | HMDB | | 2-(Acetylamino)-2-deoxy-D-galactose 4-(hydrogen sulphate) | HMDB | | GalNAc4S | HMDB | | N-Acetyl-D-galactosamine 4-sulfate | HMDB | | N-Acetyl-D-galactosamine 4-sulphate | HMDB | | N-Acetylgalactosamine-4-sulfate | HMDB | | N-Acetylgalactosamine-4-sulphate | HMDB | | NAG-4-S | HMDB | | N-[(2R,3R,4R,5R,6R)-2,4-Dihydroxy-6-(hydroxymethyl)-5-(sulfooxy)oxan-3-yl]ethanimidate | HMDB | | N-[(2R,3R,4R,5R,6R)-2,4-Dihydroxy-6-(hydroxymethyl)-5-(sulphooxy)oxan-3-yl]ethanimidate | HMDB | | N-[(2R,3R,4R,5R,6R)-2,4-Dihydroxy-6-(hydroxymethyl)-5-(sulphooxy)oxan-3-yl]ethanimidic acid | HMDB |
|
|---|
| Chemical Formula | C8H15NO9S |
|---|
| Average Molecular Weight | 301.271 |
|---|
| Monoisotopic Molecular Weight | 301.046751773 |
|---|
| IUPAC Name | [(2R,3R,4R,5R,6R)-5-acetamido-4,6-dihydroxy-2-(hydroxymethyl)oxan-3-yl]oxidanesulfonic acid |
|---|
| Traditional Name | [(2R,3R,4R,5R,6R)-5-acetamido-4,6-dihydroxy-2-(hydroxymethyl)oxan-3-yl]oxidanesulfonic acid |
|---|
| CAS Registry Number | 45233-43-0 |
|---|
| SMILES | CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@H](OS(O)(=O)=O)[C@@H]1O |
|---|
| InChI Identifier | InChI=1S/C8H15NO9S/c1-3(11)9-5-6(12)7(18-19(14,15)16)4(2-10)17-8(5)13/h4-8,10,12-13H,2H2,1H3,(H,9,11)(H,14,15,16)/t4-,5-,6-,7+,8-/m1/s1 |
|---|
| InChI Key | WHCJUIFHMJFEFZ-UIAUGNHASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as acylaminosugars. These are organic compounds containing a sugar linked to a chain through N-acyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Acylaminosugars |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acylaminosugar
- N-acyl-alpha-hexosamine
- Hexose monosaccharide
- Monosaccharide sulfate
- Monosaccharide
- Oxane
- Sulfuric acid ester
- Alkyl sulfate
- Sulfate-ester
- Sulfuric acid monoester
- Organic sulfuric acid or derivatives
- Acetamide
- Carboxamide group
- Hemiacetal
- Secondary alcohol
- Secondary carboxylic acid amide
- Oxacycle
- Carboxylic acid derivative
- Organoheterocyclic compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Alcohol
- Organic nitrogen compound
- Primary alcohol
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05gr-5690000000-9999f4a9ad628f920f6e | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0uk9-7274790000-51356389c283408fe702 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_9) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_10) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0fk9-0190000000-9873b0cf34b6a2d02305 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0059-5900000000-0a0f78bfc09ede96dc12 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-001i-9100000000-4309fcbb832ee8f8fe9d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0zgi-0095000000-9b84207cee0fb9a603c2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uec-1190000000-6ac62541fed00ea34c8d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udu-9610000000-8f949b47b6e8ba30243a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-3793000000-b4c948ad4ba7a620e6d6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9350000000-aa4efc17a9e76f1d628c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9200000000-0a15855482484fda6549 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0029000000-940928f9d0f78df7d01c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-2974000000-ccb9995db976a2f25a26 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-9810000000-822c5da05677cfbdfdd3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0uk9-0193000000-dc3a85b96a45405e85da | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0pb9-4493000000-db92c062dc52b16910d7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4j-9000000000-38775368e20d30c779e2 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|