| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:37:20 UTC |
|---|
| Update Date | 2020-04-22 15:05:33 UTC |
|---|
| BMDB ID | BMDB0000839 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
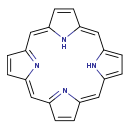
| Common Name | Pentaporphyrin I |
|---|
| Description | Pentaporphyrin I belongs to the class of organic compounds known as porphyrins. Porphyrins are compounds containing a fundamental skeleton of four pyrrole nuclei united through the alpha-positions by four methine groups to form a macrocyclic structure. Based on a literature review very few articles have been published on Pentaporphyrin I. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+)-Isoboldine | HMDB | | 2,10-Dimethoxy-6a-aporphine-1,9-diol | HMDB | | 2,10-Dimethoxy-6alpha-aporphine-1,9-diol | HMDB | | 21H,23H-Porphin | HMDB | | 21H,23H-Porphine | HMDB | | D-Isoboldine | HMDB | | delta-Isoboldine | HMDB | | Isoboldine | HMDB | | Porphine | HMDB | | Prolmon | HMDB | | Protoporphyrin disodium | HMDB |
|
|---|
| Chemical Formula | C20H14N4 |
|---|
| Average Molecular Weight | 310.352 |
|---|
| Monoisotopic Molecular Weight | 310.121846468 |
|---|
| IUPAC Name | 21,22,23,24-tetraazapentacyclo[16.2.1.1^{3,6}.1^{8,11}.1^{13,16}]tetracosa-1,3,5,7,9,11,13(22),14,16,18(21),19-undecaene |
|---|
| Traditional Name | 21,22,23,24-tetraazapentacyclo[16.2.1.1^{3,6}.1^{8,11}.1^{13,16}]tetracosa-1,3,5,7,9,11,13(22),14,16,18(21),19-undecaene |
|---|
| CAS Registry Number | 3019-51-0 |
|---|
| SMILES | N1C2=CC=C1\C=C1\C=CC(\C=C3\C=CC(\C=C4/N\C(\C=C4)=C/2)=N3)=N1 |
|---|
| InChI Identifier | InChI=1S/C20H14N4/c1-2-14-10-16-5-6-18(23-16)12-20-8-7-19(24-20)11-17-4-3-15(22-17)9-13(1)21-14/h1-12,21-22H/b13-9-,14-10-,15-9-,16-10-,17-11-,18-12-,19-11-,20-12- |
|---|
| InChI Key | JZRYQZJSTWVBBD-CEVVSZFKSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as porphyrins. Porphyrins are compounds containing a fundamental skeleton of four pyrrole nuclei united through the alpha-positions by four methine groups to form a macrocyclic structure. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrapyrroles and derivatives |
|---|
| Sub Class | Porphyrins |
|---|
| Direct Parent | Porphyrins |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03di-0009000000-c840e918f6f52a098350 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0009000000-436e5c763ea018a16e83 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0009000000-436e5c763ea018a16e83 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-0009000000-58421ad29bd1fc585e5f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0009000000-b28534cedcf9900d8448 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0009000000-b28534cedcf9900d8448 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-0029000000-f7a2f934fb7fca84713a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0009000000-89f88484153e1092dd79 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0009000000-89f88484153e1092dd79 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a59-0059000000-cae8b41cf5f8d5762556 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0009000000-cace220374bc9ffac2f6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0009000000-cace220374bc9ffac2f6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-0092000000-988e7d904ce20d755a33 | View in MoNA |
|---|
|
|---|
| Synthesis Reference | Kametani, Tetsuji; Sugahara, Tokuji; Yagi, Haruhiko; Fukumoto, Keiichiro. Synthesis of heterocyclic compounds. CCCXIV. Biogenetic type syntheses of aporphine alkaloids, isoboldine and glaucine. Tetrahedron (1969), 25(17), 3667-73. |
|---|