| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:41:51 UTC |
|---|
| Update Date | 2020-04-22 15:06:54 UTC |
|---|
| BMDB ID | BMDB0001164 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
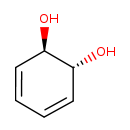
| Common Name | trans-1,2-Dihydrobenzene-1,2-diol |
|---|
| Description | trans-1,2-Dihydrobenzene-1,2-diol belongs to the class of organic compounds known as 1,2-diols. These are polyols containing an alcohol group at two adjacent positions. Based on a literature review a small amount of articles have been published on trans-1,2-Dihydrobenzene-1,2-diol. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (1R,2R)-1,2-Dihydrobenzene-1,2-diol | ChEBI | | Arene diol | HMDB | | cis-3,5-Cyclohexadiene-1,2-diol solution | HMDB | | Racemic mixture OF (+)- and (-)-1,2-dihydroxy-1,2-dihydrobenzene | HMDB | | Rel-(1R,2R)-cyclohexa-3,5-diene-1,2-diol | HMDB | | trans-(+-)-3,5-Cyclohexadiene-1,2-diol | HMDB | | trans-3,5-Cyclohexadiene-1,2-diol | HMDB | | trans-1,2-Dihydrobenzene-1,2-diol | ChEBI |
|
|---|
| Chemical Formula | C6H8O2 |
|---|
| Average Molecular Weight | 112.1265 |
|---|
| Monoisotopic Molecular Weight | 112.0524295 |
|---|
| IUPAC Name | (1R,2R)-cyclohexa-3,5-diene-1,2-diol |
|---|
| Traditional Name | arene diol |
|---|
| CAS Registry Number | 103302-38-1 |
|---|
| SMILES | O[C@@H]1C=CC=C[C@H]1O |
|---|
| InChI Identifier | InChI=1S/C6H8O2/c7-5-3-1-2-4-6(5)8/h1-8H/t5-,6-/m1/s1 |
|---|
| InChI Key | YDRSQRPHLBEPTP-PHDIDXHHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,2-diols. These are polyols containing an alcohol group at two adjacent positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Alcohols and polyols |
|---|
| Direct Parent | 1,2-diols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Secondary alcohol
- 1,2-diol
- Hydrocarbon derivative
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0zfu-9000000000-36be6170d6aeb143432b | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00xu-9710000000-3400c66fcd577a2df022 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-1900000000-d7121c202776bf3f1ff8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-5900000000-51396982b568c547b4fb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udl-9000000000-db963106694d68d1163c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0900000000-983307a7cfa91742b0da | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-1900000000-185fc58f8f3b52505cdd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ik9-9300000000-9f1f8262efe5adb2f0c9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-9000000000-e3d808ea4d2d2f328d1f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uxs-9000000000-b7cca48fb6c1e036eb1b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gbi-9000000000-569b7f3fc872051f9af9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-3900000000-e87e4c500aa5823cd259 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9100000000-6db11e4f5f30c8c63a0c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-b39a7571486a5e8d439c | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|
| Synthesis Reference | Sato, Tokuro; Fukuyama, Tomitaro; Suzuki, Taeko; Yoshikawa, Haruhisa. 1,2-Dihydro-1,2-dihydroxybenzene and several other substances in the metabolism of benzene. Journal of Biochemistry (Tokyo, Japan) (1963), 53 23-7. |
|---|