| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:49:42 UTC |
|---|
| Update Date | 2020-05-21 16:28:48 UTC |
|---|
| BMDB ID | BMDB0001993 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 7a-Hydroxy-cholestene-3-one |
|---|
| Description | 7a-Hydroxy-cholestene-3-one, also known as cholest-4-en-7alpha-ol-3-one, belongs to the class of organic compounds known as cholesterols and derivatives. Cholesterols and derivatives are compounds containing a 3-hydroxylated cholestane core. Thus, 7a-hydroxy-cholestene-3-one is considered to be a bile acid. Based on a literature review a significant number of articles have been published on 7a-Hydroxy-cholestene-3-one. |
|---|
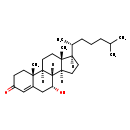
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 7alpha-Hydroxy-4-cholesten-3-one | ChEBI | | Cholest-4-en-7alpha-ol-3-one | ChEBI | | 7a-Hydroxy-4-cholesten-3-one | Generator | | 7Α-hydroxy-4-cholesten-3-one | Generator | | Cholest-4-en-7a-ol-3-one | Generator | | Cholest-4-en-7α-ol-3-one | Generator | | 7-Hydroxy-4-cholesten-3-one | MeSH | | Cholest-4-en-7 alpha-ol-3-one | MeSH | | 7 alpha-Hydroxy-4-cholesten-3-one | MeSH | | 7 alpha-3Ox-C | HMDB | | 7-a-Hydroxy-4-cholesten-3-one | HMDB | | 7-a-Hydroxycholest-4-en-3-one | HMDB | | 7-alpha-Hydroxy-4-cholesten-3-one | HMDB | | 7-alpha-Hydroxycholest-4-en-3-one | HMDB | | 7-Hydroxycholest-4-en-3-one | HMDB | | 7a-Hydroxy-4-cholesten-3-one-12alpha | HMDB | | 7alpha-Hydroxycholest-4-en-3-one | HMDB | | HCO | HMDB |
|
|---|
| Chemical Formula | C27H44O2 |
|---|
| Average Molecular Weight | 400.6371 |
|---|
| Monoisotopic Molecular Weight | 400.334130652 |
|---|
| IUPAC Name | (1S,2R,9R,10S,11S,14R,15R)-9-hydroxy-2,15-dimethyl-14-[(2R)-6-methylheptan-2-yl]tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-6-en-5-one |
|---|
| Traditional Name | 7α-hydroxy-4-cholesten-3-one |
|---|
| CAS Registry Number | 3862-25-7 |
|---|
| SMILES | [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)CC4=CC(=O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C |
|---|
| InChI Identifier | InChI=1S/C27H44O2/c1-17(2)7-6-8-18(3)21-9-10-22-25-23(12-14-27(21,22)5)26(4)13-11-20(28)15-19(26)16-24(25)29/h15,17-18,21-25,29H,6-14,16H2,1-5H3/t18-,21-,22+,23+,24-,25+,26+,27-/m1/s1 |
|---|
| InChI Key | IOIZWEJGGCZDOL-RQDYSCIWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cholesterols and derivatives. Cholesterols and derivatives are compounds containing a 3-hydroxylated cholestane core. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Cholestane steroids |
|---|
| Direct Parent | Cholesterols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cholesterol-skeleton
- 3-oxo-delta-4-steroid
- 3-oxosteroid
- 7-hydroxysteroid
- Oxosteroid
- Hydroxysteroid
- Delta-4-steroid
- Cyclohexenone
- Cyclic alcohol
- Cyclic ketone
- Secondary alcohol
- Ketone
- Organic oxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Organooxygen compound
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00dr-1339000000-8b87f8914863b4c7e8e7 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0a6r-4672900000-f5cd21522bdf7607c5cb | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f89-0019500000-764a74c3942ad452aa8e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f89-3339100000-87e6e598932d57a6274e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ab9-8469000000-b55e4f0de6f6dbfbe093 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-b03a7cbfe5ca7374d51f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0009000000-903f15d46f6bf7449623 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00lr-1009000000-fd9c44e4f27a3c62b7e8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0001900000-7ee7f5ca27c449403c35 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0pvl-9246300000-d08ac46b391d42697238 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9620000000-e3dea04967eb9e6421c1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-dc23ba87137530d83f68 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0009000000-dc23ba87137530d83f68 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-0009000000-75e53e521203e582561b | View in MoNA |
|---|
|
|---|
| Synthesis Reference | Alexander, David L.; Fisher, Jed F. A convenient synthesis of 7a-hydroxycholest-4-en-3-one by the hydroxypropyl-b-cyclodextrin-facilitated cholesterol oxidase oxidation of 3b,7a-cholest-5-ene-3,7-diol. Steroids (1995), 60(3), 290-4. |
|---|