| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:52:23 UTC |
|---|
| Update Date | 2020-04-22 15:10:06 UTC |
|---|
| BMDB ID | BMDB0002194 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
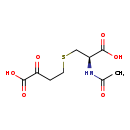
| Common Name | N-acetyl-S-(3-oxo-3-carboxy-n-propyl)cysteine |
|---|
| Description | N-acetyl-S-(3-oxo-3-carboxy-n-propyl)cysteine belongs to the class of organic compounds known as n-acyl-l-alpha-amino acids. These are n-acylated alpha amino acids which have the L-configuration of the alpha-carbon atom. Based on a literature review a significant number of articles have been published on N-acetyl-S-(3-oxo-3-carboxy-n-propyl)cysteine. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (R)-4-(2-acetamido-2-carboxyethylthio)-2-Oxobutanoate | HMDB | | (R)-4-(2-acetamido-2-carboxyethylthio)-2-Oxobutanoic acid | HMDB | | NAc-OCPC | HMDB, MeSH | | 4-{[(2R)-2-carboxy-2-[(1-hydroxyethylidene)amino]ethyl]sulfanyl}-2-oxobutanoate | Generator, HMDB | | 4-{[(2R)-2-carboxy-2-[(1-hydroxyethylidene)amino]ethyl]sulphanyl}-2-oxobutanoate | Generator, HMDB | | 4-{[(2R)-2-carboxy-2-[(1-hydroxyethylidene)amino]ethyl]sulphanyl}-2-oxobutanoic acid | Generator, HMDB | | N-Acetyl-S-(3-oxo-3-carboxy-N-propyl)cysteine | MeSH |
|
|---|
| Chemical Formula | C9H13NO6S |
|---|
| Average Molecular Weight | 263.268 |
|---|
| Monoisotopic Molecular Weight | 263.046357843 |
|---|
| IUPAC Name | 4-{[(2R)-2-carboxy-2-acetamidoethyl]sulfanyl}-2-oxobutanoic acid |
|---|
| Traditional Name | 4-{[(2R)-2-carboxy-2-acetamidoethyl]sulfanyl}-2-oxobutanoic acid |
|---|
| CAS Registry Number | 622368-00-7 |
|---|
| SMILES | CC(=O)N[C@@H](CSCCC(=O)C(O)=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C9H13NO6S/c1-5(11)10-6(8(13)14)4-17-3-2-7(12)9(15)16/h6H,2-4H2,1H3,(H,10,11)(H,13,14)(H,15,16)/t6-/m0/s1 |
|---|
| InChI Key | AHFWWWFNCBRMIV-LURJTMIESA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acyl-l-alpha-amino acids. These are n-acylated alpha amino acids which have the L-configuration of the alpha-carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | N-acyl-L-alpha-amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-acyl-l-alpha-amino acid
- Cysteine or derivatives
- Short-chain keto acid
- Thia fatty acid
- Alpha-keto acid
- Dicarboxylic acid or derivatives
- Keto acid
- Fatty acyl
- Acetamide
- Alpha-hydroxy ketone
- Carboxamide group
- Secondary carboxylic acid amide
- Ketone
- Dialkylthioether
- Sulfenyl compound
- Thioether
- Carboxylic acid
- Organic oxide
- Organonitrogen compound
- Organooxygen compound
- Organosulfur compound
- Organic nitrogen compound
- Organic oxygen compound
- Carbonyl group
- Organopnictogen compound
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9320000000-21335ec3997d84a833ff | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00r5-9744000000-9c809a5689e68bd0dcea | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0il1-1590000000-6940905073c10894c7d0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03l1-4920000000-e0e63094e181793e90b1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0089-9500000000-5ccba883f4b5ca55b9aa | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-1690000000-42488e6d5ae9973a6d71 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03el-4920000000-a3213f11944fbb140598 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4r-9500000000-c1314663a0d84af4d149 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0400-0900000000-041fd2bf9951a1653247 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fur-2900000000-f1f95f7c7927959ed8b1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9000000000-895719d37ece37a5b796 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0790000000-44ca69fa43c4178bf836 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a59-9700000000-9ede141cb38dc7cb2f0a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9200000000-f838fe566f276209ec40 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|
| Synthesis Reference | Pankau, Wolf Matthias; Moenninghoff, Sven; von Kiedrowski, Guenter. Thermostable and monoconjugable gold clusters with a dodecadentate thioether ligand gripper. Angewandte Chemie, International Edition (2006), 45(12), 1889-1891. |
|---|