| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:52:55 UTC |
|---|
| Update Date | 2020-06-04 20:50:36 UTC |
|---|
| BMDB ID | BMDB0002226 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Adrenic acid |
|---|
| Description | Adrenic acid, also known as adrenate, belongs to the class of organic compounds known as very long-chain fatty acids. These are fatty acids with an aliphatic tail that contains at least 22 carbon atoms. Adrenic acid is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. Adrenic acid participates in a number of enzymatic reactions, within cattle. In particular, Adrenic acid can be biosynthesized from arachidonic acid through its interaction with the enzyme elongation OF very long chain fatty acids protein 5. In addition, Adrenic acid can be converted into tetracosatetraenoic acid (24:4N-6); which is mediated by the enzyme elongation OF very long chain fatty acids protein 4. In cattle, adrenic acid is involved in the metabolic pathway called the Alpha linolenic Acid and linoleic Acid metabolism pathway. |
|---|
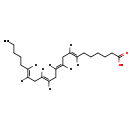
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Adrenate | Generator | | Adrenic acid, (Z)-isomer | MeSH | | 7,10,13,16-Docosatetraenoic acid | MeSH | | Adrenic acid | MeSH |
|
|---|
| Chemical Formula | C22H36O2 |
|---|
| Average Molecular Weight | 332.528 |
|---|
| Monoisotopic Molecular Weight | 332.271530399 |
|---|
| IUPAC Name | (7E,10E,13E,16E)-docosa-7,10,13,16-tetraenoic acid |
|---|
| Traditional Name | adrenate |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H]\C(CCCCC)=C(\[H])C\C([H])=C(/[H])C\C([H])=C(/[H])C\C([H])=C(/[H])CCCCCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C22H36O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22(23)24/h6-7,9-10,12-13,15-16H,2-5,8,11,14,17-21H2,1H3,(H,23,24)/b7-6+,10-9+,13-12+,16-15+ |
|---|
| InChI Key | TWSWSIQAPQLDBP-CGRWFSSPSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as very long-chain fatty acids. These are fatty acids with an aliphatic tail that contains at least 22 carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
| Direct Parent | Very long-chain fatty acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Very long-chain fatty acid
- Unsaturated fatty acid
- Straight chain fatty acid
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Adiposome
- Cell membrane
- Cytoplasm
- Membrane
- Myelin sheath
- Peroxisome
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | |
|---|
| General References | - Glasser F, Ferlay A, Chilliard Y: Oilseed lipid supplements and fatty acid composition of cow milk: a meta-analysis. J Dairy Sci. 2008 Dec;91(12):4687-703. doi: 10.3168/jds.2008-0987. [PubMed:19038946 ]
- M. Ferrand, B. Huquet. S. Barbey, F. Barillet, F. Faucon, H. Larroque, O. Leray, J.M. Trommenschlager, M. Brochard (2011). M. Ferrand et al. Determination of fatty acid profile in cow's milk using mid-infrared spectrometry: Interest of applying a variable selection by genetic algorithms before a PLS regression. Chemometrics and Intelligent Laboratory Systems 106 (2011) 183?189. Chemometrics and Intelligent Laboratory Systems.
|
|---|