| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:53:37 UTC |
|---|
| Update Date | 2020-04-22 15:10:31 UTC |
|---|
| BMDB ID | BMDB0002285 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
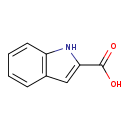
| Common Name | 2-Indolecarboxylic acid |
|---|
| Description | 2-Indolecarboxylic acid, also known as indol-2-carboxylate or 2-carboxyindole, belongs to the class of organic compounds known as indolecarboxylic acids and derivatives. Indolecarboxylic acids and derivatives are compounds containing a carboxylic acid group (or a derivative thereof) linked to an indole. 2-Indolecarboxylic acid is possibly soluble (in water) and a weakly acidic compound (based on its pKa). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Indolecarboxylate | Generator | | indol-2-Carboxylic acid | ChEMBL, HMDB | | indol-2-Carboxylate | Generator, HMDB | | 1H-Indole-2-carboxylate | HMDB, Generator | | 1H-Indole-2-carboxylic acid | HMDB | | 2-Carboxyindole | HMDB | | Indole-2-carboxylate | HMDB | | Indole-2-carboxylic acid | HMDB, MeSH |
|
|---|
| Chemical Formula | C9H7NO2 |
|---|
| Average Molecular Weight | 161.1574 |
|---|
| Monoisotopic Molecular Weight | 161.047678473 |
|---|
| IUPAC Name | 1H-indole-2-carboxylic acid |
|---|
| Traditional Name | indole-2-carboxylic acid |
|---|
| CAS Registry Number | 1477-50-5 |
|---|
| SMILES | OC(=O)C1=CC2=C(N1)C=CC=C2 |
|---|
| InChI Identifier | InChI=1S/C9H7NO2/c11-9(12)8-5-6-3-1-2-4-7(6)10-8/h1-5,10H,(H,11,12) |
|---|
| InChI Key | HCUARRIEZVDMPT-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as indolecarboxylic acids and derivatives. Indolecarboxylic acids and derivatives are compounds containing a carboxylic acid group (or a derivative thereof) linked to an indole. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Indoles and derivatives |
|---|
| Sub Class | Indolecarboxylic acids and derivatives |
|---|
| Direct Parent | Indolecarboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Indolecarboxylic acid derivative
- Indole
- Pyrrole-2-carboxylic acid
- Pyrrole-2-carboxylic acid or derivatives
- Substituted pyrrole
- Benzenoid
- Pyrrole
- Heteroaromatic compound
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Azacycle
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | 2.31 | HANSCH,C ET AL. (1995) |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01ox-4900000000-71da0f4b4d797e27273b | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00y0-9770000000-1d97508714d0fc338220 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0900000000-f4554fa32e1dc60685f1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0900000000-4e4ee101de19b55dc5ff | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-1900000000-1568880a7641c6067190 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0900000000-06e55ebe614b04ed5f75 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03xr-0900000000-958b289f26a6c0a5c47d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014l-2900000000-6698c24dd617648873c9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0900000000-70e9cda69dd6169dc2a8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01ox-0900000000-882a9335e9dcffc135e2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014l-3900000000-975d31c56ec06b8fadb1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0900000000-89c3f30c8225ba6d5d62 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0900000000-89c3f30c8225ba6d5d62 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-0900000000-89c3f30c8225ba6d5d62 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|
| General References | - Qian L, Zhao A, Zhang Y, Chen T, Zeisel SH, Jia W, Cai W: Metabolomic Approaches to Explore Chemical Diversity of Human Breast-Milk, Formula Milk and Bovine Milk. Int J Mol Sci. 2016 Dec 17;17(12). pii: ijms17122128. doi: 10.3390/ijms17122128. [PubMed:27999311 ]
|
|---|