| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:53:55 UTC |
|---|
| Update Date | 2020-04-22 15:10:36 UTC |
|---|
| BMDB ID | BMDB0002308 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Hydroxycobalamin |
|---|
| Description | Hydroxycobalamin belongs to the class of organic compounds known as cobalamin derivatives. These are organic compounds containing a corrin ring, a cobalt atom, an a nucleotide moiety. Cobalamin Derivatives are actually derived from vitamin B12. Hydroxycobalamin is a very strong basic compound (based on its pKa). |
|---|
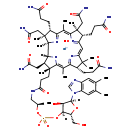
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Hydroxocobalamin | MeSH | | Hydroxo-cobalamin | MeSH | | a-(5,6-Dimethylbenzimidazolyl)hydroxocobamide | HMDB | | alpha Cobione | HMDB | | alpha-(5,6-Dimethylbenzimidazolyl)hydroxocobamide | HMDB | | Axlon | HMDB | | Ciplamin H | HMDB | | Cobalex | HMDB | | Cobalin H | HMDB | | Cobinamide hydroxide phosphate 3'-ester with 5,6-dimethyl-1-a-D-ribofuranosylbenzimidazole inner salt | HMDB | | Cobinamide hydroxide phosphate 3'-ester with 5,6-dimethyl-1-alpha-delta-ribofuranosylbenzimidazole inner salt | HMDB | | Codroxomin | HMDB | | Docclan | HMDB | | Docelan | HMDB | | Docevita | HMDB | | Droxomin | HMDB | | Ducobee hy | HMDB | | Duradoce | HMDB | | Duralta-12 | HMDB | | Hydrocobalamin | HMDB | | Hydrogrisevit | HMDB | | Hydrovit | HMDB | | Hydroxocobalamine | HMDB | | -cobalt(2+) ion 1-[(2S,3R,4S,5R)-3-hydroxy-4-{[hydroxy({[(2R)-1-({1-hydroxy-3-[(1R,2R,3R,4R,8S,13S,14S,18S,19S)-8,13,18-tris(2-carboximidatoethyl)-3,14,19-tris[(C-hydroxycarbonimidoyl)methyl]-1,4,6,9,9,14,16,19-octamethyl-20,21,22,23-tetraazapentacyclo[15.2.1.1,.1,.1,]tricosa-5(23),6,10(22),11,15(21),16-hexaen-4-yl]propylidene}amino)propan-2-yl]oxy})phosphoryl]oxy}-5-(hydroxymethyl)oxolan-2-yl]-5,6-dimethyl-3H-1,3-benzodiazol-1-ylium hydric acid | Generator |
|
|---|
| Chemical Formula | C62H90CoN13O15P |
|---|
| Average Molecular Weight | 1347.3631 |
|---|
| Monoisotopic Molecular Weight | 1346.574895981 |
|---|
| IUPAC Name | lambda2-cobalt(2+) ion (1R,2R,3S,4S,8S,9S,14S,18R,19R)-4,9,14-tris(2-carbamoylethyl)-3,8,19-tris(carbamoylmethyl)-18-(2-{[(2R)-2-{[(2R,3S,4R,5S)-5-(5,6-dimethyl-1H-1,3-benzodiazol-1-yl)-4-hydroxy-2-(hydroxymethyl)oxolan-3-yl phosphono]oxy}propyl]carbamoyl}ethyl)-2,3,6,8,13,13,16,18-octamethyl-20,21,22,23-tetraazapentacyclo[15.2.1.1^{2,5}.1^{7,10}.1^{12,15}]tricosa-5(23),6,10(22),11,15(21),16-hexaen-20-ide hydrate |
|---|
| Traditional Name | lambda2-cobalt(2+) ion (1R,2R,3S,4S,8S,9S,14S,18R,19R)-4,9,14-tris(2-carbamoylethyl)-3,8,19-tris(carbamoylmethyl)-18-(2-{[(2R)-2-{[(2R,3S,4R,5S)-5-(5,6-dimethyl-1,3-benzodiazol-1-yl)-4-hydroxy-2-(hydroxymethyl)oxolan-3-yl phosphono]oxy}propyl]carbamoyl}ethyl)-2,3,6,8,13,13,16,18-octamethyl-20,21,22,23-tetraazapentacyclo[15.2.1.1^{2,5}.1^{7,10}.1^{12,15}]tricosa-5(23),6,10(22),11,15(21),16-hexaen-20-ide hydrate |
|---|
| CAS Registry Number | 13422-51-0 |
|---|
| SMILES | O.[Co++].[H][C@@]1(OP([O-])(=O)O[C@H](C)CNC(=O)CC[C@]2(C)[C@@H](CC(N)=O)[C@@]3([H])[N-]\C2=C(C)/C2=N/C(=C\C4=N\C(=C(C)/C5=N[C@]3(C)[C@@](C)(CC(N)=O)[C@@H]5CCC(N)=O)\[C@@](C)(CC(N)=O)[C@@H]4CCC(N)=O)/C(C)(C)[C@@H]2CCC(N)=O)[C@@H](CO)O[C@@]([H])([C@@H]1O)N1C=NC2=C1C=C(C)C(C)=C2 |
|---|
| InChI Identifier | InChI=1S/C62H90N13O14P.Co.H2O/c1-29-20-39-40(21-30(29)2)75(28-70-39)57-52(84)53(41(27-76)87-57)89-90(85,86)88-31(3)26-69-49(83)18-19-59(8)37(22-46(66)80)56-62(11)61(10,25-48(68)82)36(14-17-45(65)79)51(74-62)33(5)55-60(9,24-47(67)81)34(12-15-43(63)77)38(71-55)23-42-58(6,7)35(13-16-44(64)78)50(72-42)32(4)54(59)73-56;;/h20-21,23,28,31,34-37,41,52-53,56-57,76,84H,12-19,22,24-27H2,1-11H3,(H15,63,64,65,66,67,68,69,71,72,73,74,77,78,79,80,81,82,83,85,86);;1H2/q;+2;/p-2/t31-,34-,35-,36-,37+,41-,52-,53-,56-,57+,59-,60+,61+,62+;;/m1../s1 |
|---|
| InChI Key | DQOCFCZRZOAIBN-WZHZPDAFSA-L |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cobalamin derivatives. These are organic compounds containing a corrin ring, a cobalt atom, an a nucleotide moiety. Cobalamin Derivatives are actually derived from vitamin B12. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrapyrroles and derivatives |
|---|
| Sub Class | Corrinoids |
|---|
| Direct Parent | Cobalamin derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cobalamin
- Hydroxycobalamin
- Metallotetrapyrrole skeleton
- 1-ribofuranosylbenzimidazole
- Pentose phosphate
- N-glycosyl compound
- Glycosyl compound
- Monosaccharide phosphate
- Pentose monosaccharide
- Benzimidazole
- Phosphoethanolamine
- Dialkyl phosphate
- Fatty acyl
- Fatty amide
- Monosaccharide
- N-substituted imidazole
- Benzenoid
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Imidazole
- Heteroaromatic compound
- Azole
- Pyrrolidine
- Pyrroline
- Tetrahydrofuran
- Carboxamide group
- Primary carboxylic acid amide
- Secondary alcohol
- Secondary carboxylic acid amide
- Ketimine
- Azacycle
- Oxacycle
- Carbene-type 1,3-dipolar compound
- Carboxylic acid derivative
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Organic transition metal salt
- Alcohol
- Organonitrogen compound
- Imine
- Organooxygen compound
- Primary alcohol
- Organic zwitterion
- Organic salt
- Organic cobalt salt
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Carbonyl group
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-000i-2120000009-a1a6c934c09c0655b549 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0f79-4240000029-c4f23872a45ca99d454c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0f79-5240000139-aa3cc38ae6fc78c5c9d7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-1404000009-d95c227e0c2a01a825ad | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-2902000005-61bd391f47e0b9c2ba4b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-3930000015-9f00c742a0c9e52997c4 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, CD3OD, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|