| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:00:30 UTC |
|---|
| Update Date | 2020-04-22 15:11:04 UTC |
|---|
| BMDB ID | BMDB0002496 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
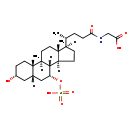
| Common Name | N-[(3a,5b,7a)-3-hydroxy-24-oxo-7-(sulfooxy)cholan-24-yl]-Glycine |
|---|
| Description | N-[(3a,5b,7a)-3-hydroxy-24-oxo-7-(sulfooxy)cholan-24-yl]-Glycine, also known as N-(3alpha-hydroxy-7alpha-sulfooxy-5beta-cholan-24-oyl)glycine, belongs to the class of organic compounds known as glycinated bile acids and derivatives. Glycinated bile acids and derivatives are compounds with a structure characterized by the presence of a glycine linked to a bile acid skeleton. Thus, N-[(3a,5b,7a)-3-hydroxy-24-oxo-7-(sulfooxy)cholan-24-yl]-glycine is considered to be a steroid conjugate. Based on a literature review very few articles have been published on N-[(3a,5b,7a)-3-hydroxy-24-oxo-7-(sulfooxy)cholan-24-yl]-Glycine. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| N-(3alpha-Hydroxy-7alpha-sulfooxy-5beta-cholan-24-oyl)glycine | ChEBI | | N-(3a-Hydroxy-7a-sulfooxy-5b-cholan-24-oyl)glycine | Generator | | N-(3a-Hydroxy-7a-sulphooxy-5b-cholan-24-oyl)glycine | Generator | | N-(3alpha-Hydroxy-7alpha-sulphooxy-5beta-cholan-24-oyl)glycine | Generator | | N-(3Α-hydroxy-7α-sulfooxy-5β-cholan-24-oyl)glycine | Generator | | N-(3Α-hydroxy-7α-sulphooxy-5β-cholan-24-oyl)glycine | Generator | | N-[(3a,5b,7a)-3-Hydroxy-24-oxo-7-(sulphooxy)cholan-24-yl]-glycine | Generator | | Glycochenodeoxycholate 7-sulfate | HMDB, Generator | | Glycochenodeoxycholate 7-sulphate | HMDB, Generator | | Glycochenodeoxycholic acid 7-sulfate | HMDB | | Glycochenodeoxycholic acid 7-sulphate | HMDB | | Glycochenodeoxycholyl 7-sulfate | HMDB | | Glycochenodeoxycholyl 7-sulphate | HMDB | | Glycochenodeoxycholic acid 7-sulfuric acid | Generator, HMDB | | Glycochenodeoxycholic acid 7-sulphuric acid | Generator, HMDB |
|
|---|
| Chemical Formula | C26H43NO8S |
|---|
| Average Molecular Weight | 529.687 |
|---|
| Monoisotopic Molecular Weight | 529.270938047 |
|---|
| IUPAC Name | 2-[(4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R)-5-hydroxy-2,15-dimethyl-9-(sulfooxy)tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-yl]pentanamido]acetic acid |
|---|
| Traditional Name | [(4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R)-5-hydroxy-2,15-dimethyl-9-(sulfooxy)tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-yl]pentanamido]acetic acid |
|---|
| CAS Registry Number | 67030-55-1 |
|---|
| SMILES | [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)OS(O)(=O)=O)[C@H](C)CCC(=O)NCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C26H43NO8S/c1-15(4-7-22(29)27-14-23(30)31)18-5-6-19-24-20(9-11-26(18,19)3)25(2)10-8-17(28)12-16(25)13-21(24)35-36(32,33)34/h15-21,24,28H,4-14H2,1-3H3,(H,27,29)(H,30,31)(H,32,33,34)/t15-,16+,17-,18-,19+,20+,21-,24+,25+,26-/m1/s1 |
|---|
| InChI Key | GLYPHOJMMLQNJQ-GYPHWSFCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as glycinated bile acids and derivatives. Glycinated bile acids and derivatives are compounds with a structure characterized by the presence of a glycine linked to a bile acid skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
| Direct Parent | Glycinated bile acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Glycinated bile acid
- Hydroxy bile acid, alcohol, or derivatives
- Monohydroxy bile acid, alcohol, or derivatives
- Sulfated steroid skeleton
- 3-hydroxysteroid
- Hydroxysteroid
- 3-alpha-hydroxysteroid
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid or derivatives
- Fatty amide
- Fatty acyl
- N-acyl-amine
- Sulfuric acid ester
- Alkyl sulfate
- Sulfate-ester
- Sulfuric acid monoester
- Organic sulfuric acid or derivatives
- Cyclic alcohol
- Carboxamide group
- Secondary carboxylic acid amide
- Secondary alcohol
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carbonyl group
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organonitrogen compound
- Organooxygen compound
- Alcohol
- Organic oxygen compound
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0nt9-1211950000-240f1393275d0bac60f0 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0a4i-2101029000-bba999add02a63a5ade4 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("N-[(3a,5b,7a)-3-hydroxy-24-oxo-7-(sulfooxy)cholan-24-yl]-Glycine,1TMS,#1" TMS) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03e9-4000890000-ce4f58a5852e63ef500d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0059-8002900000-45211dfeaddffb7ceabe | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-9004200000-f6a2ab7ebcea4fd6a951 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000490000-677a58b1c1864daa52b8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-008a-3000920000-f2cab07a2ae7567da387 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-9101100000-02088e18627c221a62ef | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000090000-47108d59914be103681d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01t9-4000980000-f0f78192720fd9ab5ea6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00fr-9000220000-835a84594b88cd9fdeb0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0001490000-9c8c9ba0b4d069fd40a8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-1004910000-731d52c3c088f18e6273 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4m-9341400000-a24f7508c4b762026cb0 | View in MoNA |
|---|
|
|---|