| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:01:39 UTC |
|---|
| Update Date | 2020-05-11 20:57:10 UTC |
|---|
| BMDB ID | BMDB0002802 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Cortisone |
|---|
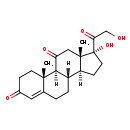
| Description | Cortisonee, also known as cortisone or cortisone, belongs to the class of organic compounds known as 21-hydroxysteroids. These are steroids carrying a hydroxyl group at the 21-position of the steroid backbone. Thus, cortisonee is considered to be a steroid lipid molecule. Cortisonee exists as a solid, very hydrophobic, practically insoluble (in water), and relatively neutral molecule. Cortisonee participates in a number of enzymatic reactions, within cattle. In particular, Cortisonee can be biosynthesized from 17a,21-dihydroxy-5b-pregnane-3,11,20-trione; which is mediated by the enzyme 3-oxo-5-beta-steroid 4-dehydrogenase. In addition, Cortisonee, nadph, and hydrogen ion can be biosynthesized from cortisol and nadp through its interaction with the enzyme corticosteroid 11-beta-dehydrogenase isozyme 2. In cattle, cortisonee is involved in the metabolic pathway called the steroidogenesis pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 11-Dehydro-17-hydroxycorticosterone | ChEBI | | 17-Hydroxy-11-dehydrocorticosterone | ChEBI | | 17alpha,21-Dihydroxy-4-pregnene-3,11,20-trione | ChEBI | | 4-Pregnene-17alpha,21-diol-3,11,20-trione | ChEBI | | Cortison | ChEBI | | Delta(4)-Pregnene-17alpha,21-diol-3,11,20-trione | ChEBI | | Kendall's compound e | ChEBI | | Kortison | ChEBI | | Pregn-4-en-17alpha,21-diol-3,11,20-trione | ChEBI | | Reichstein's substance fa | ChEBI | | Wintersteiner's compound F | ChEBI | | 17a,21-Dihydroxy-4-pregnene-3,11,20-trione | Generator | | 17Α,21-dihydroxy-4-pregnene-3,11,20-trione | Generator | | 4-Pregnene-17a,21-diol-3,11,20-trione | Generator | | 4-Pregnene-17α,21-diol-3,11,20-trione | Generator | | delta(4)-Pregnene-17a,21-diol-3,11,20-trione | Generator | | Δ(4)-pregnene-17α,21-diol-3,11,20-trione | Generator | | Pregn-4-en-17a,21-diol-3,11,20-trione | Generator | | Pregn-4-en-17α,21-diol-3,11,20-trione | Generator | | Δ(4)-pregnene-17a,21-diol-3,11,20-trione | HMDB | | Andreson | HMDB | | Anusol HC | HMDB | | Balneol-HC | HMDB | | beta-HC | HMDB | | Colocort | HMDB | | Compound e | HMDB | | Corlin | HMDB | | Cortadren | HMDB | | Cortandren | HMDB | | Cortef | HMDB | | Cortef acetate | HMDB | | Cortisal | HMDB | | Cortisate | HMDB | | Cortisone acetate | HMDB | | Cortistal | HMDB | | Cortivite | HMDB | | Cortogen | HMDB | | Cortone | HMDB | | Cortril | HMDB | | Dermacort | HMDB | | Dricort | HMDB | | Flexicort | HMDB | | Florinef | HMDB | | Fludrocortisone acetate | HMDB | | Glycort | HMDB | | Hemsol-HC | HMDB | | Hi-cor | HMDB | | Incortin | HMDB | | Kendall'S compound | HMDB | | Locoid | HMDB | | Locoid lipocream | HMDB | | Micort-HC | HMDB | | Nogenic HC | HMDB | | Orabase hca | HMDB | | Pandel | HMDB | | Prestwick_132 | HMDB | | Reichstein fa | HMDB | | Scheroson | HMDB | | Solu-cortef | HMDB | | Stie-cort | HMDB | | Texacort | HMDB | | Westcort | HMDB | | Adreson | HMDB | | Cortone acetate | HMDB | | 17-Hydroxy-3,11,20-trioxopregn-4-en-21-yl acetate | HMDB |
|
|---|
| Chemical Formula | C21H28O5 |
|---|
| Average Molecular Weight | 360.444 |
|---|
| Monoisotopic Molecular Weight | 360.193674006 |
|---|
| IUPAC Name | (1S,2R,10S,11S,14R,15S)-14-hydroxy-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-ene-5,17-dione |
|---|
| Traditional Name | (1S,2R,10S,11S,14R,15S)-14-hydroxy-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-ene-5,17-dione |
|---|
| CAS Registry Number | 53-06-5 |
|---|
| SMILES | [H][C@@]12CC[C@](O)(C(=O)CO)[C@@]1(C)CC(=O)[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C21H28O5/c1-19-7-5-13(23)9-12(19)3-4-14-15-6-8-21(26,17(25)11-22)20(15,2)10-16(24)18(14)19/h9,14-15,18,22,26H,3-8,10-11H2,1-2H3/t14-,15-,18+,19-,20-,21-/m0/s1 |
|---|
| InChI Key | MFYSYFVPBJMHGN-ZPOLXVRWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 21-hydroxysteroids. These are steroids carrying a hydroxyl group at the 21-position of the steroid backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Hydroxysteroids |
|---|
| Direct Parent | 21-hydroxysteroids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Progestogin-skeleton
- 21-hydroxysteroid
- Pregnane-skeleton
- 20-oxosteroid
- 3-oxo-delta-4-steroid
- 3-oxosteroid
- 17-hydroxysteroid
- Oxosteroid
- 11-oxosteroid
- Delta-4-steroid
- Cyclohexenone
- Alpha-hydroxy ketone
- Cyclic alcohol
- Tertiary alcohol
- Cyclic ketone
- Ketone
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organic oxygen compound
- Organooxygen compound
- Primary alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Endoplasmic reticulum
- Membrane
- Myelin sheath
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 222 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 0.28 mg/mL at 25 °C | Not Available | | LogP | 1.47 | HANSCH,C ET AL. (1995) |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00kf-2910000000-da435cee921dd7b9f8e6 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00kf-2910000000-da435cee921dd7b9f8e6 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05ai-5879000000-d8ce750671bf51b487e2 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0f79-2918800000-ab308a6a5023c34bc22f | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-03di-0009000000-5d34de066e82cc9f0a41 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-03di-0900000000-24ec04976da03c3f9c77 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0fdo-4900000000-d0880bfc6f3d80e4aa72 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-03di-3940000000-16be8720b1c49cea9b06 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-004i-0009000000-6c22c083c2dfab97a299 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-004i-0109000000-48c96b3d1471c5cd6171 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-002r-0914000000-2466a9146383b4265e42 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-000i-0900000000-214430a00501054cc774 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-059j-1900000000-0e23516e07f0baf74c79 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-0abj-1900000000-ad0282ab7188d9ec3dc1 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-0aba-3900000000-e36e464b157b3e6159f1 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-0a4j-5900000000-fe9092d76099e1e70a4e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-0aou-6900000000-1b218b97a890ac7e8f45 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , negative | splash10-03fr-0049000000-3bd424d473bdadd2b9b4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , negative | splash10-03di-0039000000-fd181dfb87e1cbb8ab6f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , negative | splash10-03di-0049000000-7633496a1a53bda21707 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , negative | splash10-004i-0009000000-59db5e627634e4be3907 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , negative | splash10-004i-0009000000-9781e204df305d722d2d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , negative | splash10-004i-0009000000-0b5e40226163a1a334db | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03dl-0009000000-946472abc72d41b1633c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-003u-0149000000-efb3f94efcbdfc468859 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a5j-2392000000-d014016e46f2ae8b1bd1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0009000000-1c4a8c3698ff1bfa2bd9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0pb9-2029000000-82b546174619108311f8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ab9-8093000000-47f7996c84edb1324aad | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, 100%_DMSO, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, 100%_DMSO, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|