| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:02:07 UTC |
|---|
| Update Date | 2020-04-22 15:11:31 UTC |
|---|
| BMDB ID | BMDB0002904 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 2,3-Dinor-TXB2 |
|---|
| Description | 2,3-Dinor-TXB2, also known as 2,3-dinor TXB2, belongs to the class of organic compounds known as thromboxanes. These are eicosanoids structurally characterized by the presence of a 6-member ether containing ring. Thus, 2,3-dinor-TXB2 is considered to be an eicosanoid. Based on a literature review a significant number of articles have been published on 2,3-Dinor-TXB2. |
|---|
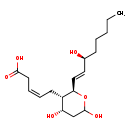
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,3-Dinor-thromboxane | HMDB | | 2,3-Dinorthromboxane | HMDB | | 2,3-Dinor TXB2 | HMDB | | 2,3-Dinor-thromboxane b2 | HMDB |
|
|---|
| Chemical Formula | C18H30O6 |
|---|
| Average Molecular Weight | 342.4272 |
|---|
| Monoisotopic Molecular Weight | 342.204238692 |
|---|
| IUPAC Name | (3Z)-5-[(2R,3S,4S)-4,6-dihydroxy-2-[(1E,3S)-3-hydroxyoct-1-en-1-yl]oxan-3-yl]pent-3-enoic acid |
|---|
| Traditional Name | 2,3-dinor-thromboxane |
|---|
| CAS Registry Number | 63250-09-9 |
|---|
| SMILES | CCCCC[C@H](O)\C=C\[C@H]1OC(O)C[C@H](O)[C@@H]1C\C=C/CC(O)=O |
|---|
| InChI Identifier | InChI=1S/C18H30O6/c1-2-3-4-7-13(19)10-11-16-14(8-5-6-9-17(21)22)15(20)12-18(23)24-16/h5-6,10-11,13-16,18-20,23H,2-4,7-9,12H2,1H3,(H,21,22)/b6-5-,11-10+/t13-,14-,15-,16+,18?/m0/s1 |
|---|
| InChI Key | RJHNVFKNIJQTQF-LMIBIYGPSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as thromboxanes. These are eicosanoids structurally characterized by the presence of a 6-member ether containing ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Eicosanoids |
|---|
| Direct Parent | Thromboxanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Thromboxane
- Medium-chain hydroxy acid
- Medium-chain fatty acid
- Heterocyclic fatty acid
- Hydroxy fatty acid
- Oxane
- Fatty acid
- Unsaturated fatty acid
- Hemiacetal
- Secondary alcohol
- Oxacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organoheterocyclic compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Alcohol
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-8983000000-6670f464141af0be2e50 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-014i-4400179000-20a281a734fc918affa0 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-056r-0359000000-5f9e6cceab7c3f7a9cbd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-056r-4196000000-fdbaed01f5494e2612fb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0lkc-9200000000-e8fea20e7b1476c8517d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00kf-0096000000-fe2510f0641448d03e95 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-010c-2895000000-da0962ceb32a19fafd35 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9550000000-a56f9116ee1c3deadd2f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0009000000-f06a87e4cd9775888635 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-3069000000-a9c4ca81a902cb920d57 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00ku-9680000000-072c422c4b7e434204e2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0029000000-86f656cdda4b71fa4cdd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9314000000-c05525088475e906eac5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9500000000-360bebc4fdec8cafc418 | View in MoNA |
|---|
|
|---|