| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:02:12 UTC |
|---|
| Update Date | 2020-05-21 16:29:06 UTC |
|---|
| BMDB ID | BMDB0002935 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | cis-b,b-Carotene |
|---|
| Description | Beta-Carotene, also known as B-carotene, belongs to the class of organic compounds known as carotenes. These are a type of unsaturated hydrocarbons containing eight consecutive isoprene units. They are characterized by the presence of two end-groups (mostly cyclohexene rings, but also cyclopentene rings or acyclic groups) linked by a long branched alkyl chain. Carotenes belonging form a subgroup of the carotenoids family. Beta-Carotene is possibly soluble (in water) and possibly neutral. Beta-Carotene exists in all living species, ranging from bacteria to humans. |
|---|
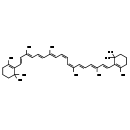
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| b-Carotene | Generator | | Β-carotene | Generator, HMDB |
|
|---|
| Chemical Formula | C40H56 |
|---|
| Average Molecular Weight | 536.8726 |
|---|
| Monoisotopic Molecular Weight | 536.438201792 |
|---|
| IUPAC Name | 1,3,3-trimethyl-2-[(1E,3E,5E,7E,9Z,11Z,13E,15E,17E)-3,7,12,16-tetramethyl-18-(2,6,6-trimethylcyclohex-1-en-1-yl)octadeca-1,3,5,7,9,11,13,15,17-nonaen-1-yl]cyclohex-1-ene |
|---|
| Traditional Name | 1,3,3-trimethyl-2-[(1E,3E,5E,7E,9Z,11Z,13E,15E,17E)-3,7,12,16-tetramethyl-18-(2,6,6-trimethylcyclohex-1-en-1-yl)octadeca-1,3,5,7,9,11,13,15,17-nonaen-1-yl]cyclohex-1-ene |
|---|
| CAS Registry Number | 30430-49-0 |
|---|
| SMILES | C\C(\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C)=C\C=C/C=C(\C)/C=C/C=C(\C)/C=C/C1=C(C)CCCC1(C)C |
|---|
| InChI Identifier | InChI=1S/C40H56/c1-31(19-13-21-33(3)25-27-37-35(5)23-15-29-39(37,7)8)17-11-12-18-32(2)20-14-22-34(4)26-28-38-36(6)24-16-30-40(38,9)10/h11-14,17-22,25-28H,15-16,23-24,29-30H2,1-10H3/b12-11-,19-13+,20-14+,27-25+,28-26+,31-17-,32-18+,33-21+,34-22+ |
|---|
| InChI Key | OENHQHLEOONYIE-KBPBZLIPSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as carotenes. These are a type of unsaturated hydrocarbons containing eight consecutive isoprene units. They are characterized by the presence of two end-groups (mostly cyclohexene rings, but also cyclopentene rings or acyclic groups) linked by a long branched alkyl chain. Carotenes belonging form a subgroup of the carotenoids family. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Tetraterpenoids |
|---|
| Direct Parent | Carotenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Carotene
- Branched unsaturated hydrocarbon
- Cycloalkene
- Cyclic olefin
- Unsaturated aliphatic hydrocarbon
- Unsaturated hydrocarbon
- Olefin
- Hydrocarbon
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-3211290000-f968c686d39ae0f0fc50 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0016-2794670000-c5bc2a4dbcd0f12cbf7f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0002-2687970000-782d048d873c21fc6b82 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-05ai-1009510000-585581456b10671692bd | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - FAB-EBEB (JMS-HX/HX 110A, JEOL) , Positive | splash10-05mx-2920010000-471219f3e66bafb29bee | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0006-0144900000-2fa7b609c7300ff8aba3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0534290000-556febfe6f0feaffd959 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0012-0967200000-81bf905ffd7943cfc997 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000j-3869300000-bce59dcd4ea231326003 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000090000-300e8e4a326ba007573b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0000090000-3ea2a05dae2343b7708f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014r-0968170000-f18c08aac9d12ac133d7 | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-05mx-9810010000-9a261fb8602890c2481e | View in MoNA |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, CDCl3, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|