| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:02:32 UTC |
|---|
| Update Date | 2020-05-11 20:23:30 UTC |
|---|
| BMDB ID | BMDB0003036 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Triiodothyronine sulfate |
|---|
| Description | Triiodothyronine sulfate belongs to the class of organic compounds known as phenylalanine and derivatives. Phenylalanine and derivatives are compounds containing phenylalanine or a derivative thereof resulting from reaction of phenylalanine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. Based on a literature review very few articles have been published on Triiodothyronine sulfate. |
|---|
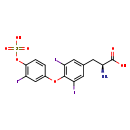
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2S)-2-Amino-3-{3,5-diiodo-4-[3-iodo-4-(sulfooxy)phenoxy]phenyl}propanoic acid | ChEBI | | Triiodothyronine sulfuric ester | ChEBI | | (2S)-2-Amino-3-{3,5-diiodo-4-[3-iodo-4-(sulfooxy)phenoxy]phenyl}propanoate | Generator | | (2S)-2-Amino-3-{3,5-diiodo-4-[3-iodo-4-(sulphooxy)phenoxy]phenyl}propanoate | Generator | | (2S)-2-Amino-3-{3,5-diiodo-4-[3-iodo-4-(sulphooxy)phenoxy]phenyl}propanoic acid | Generator | | Triiodothyronine sulphuric ester | Generator | | Triiodothyronine sulfuric acid | Generator | | Triiodothyronine sulphate | Generator | | Triiodothyronine sulphuric acid | Generator | | 3,5,3'-triiodo-L-Thyronine 4'-O-sulfate | HMDB | | 3,5,3'-triiodo-L-Thyronine 4'-O-sulphate | HMDB | | 3,5,3'-triiodo-L-Thyronine 4-O-sulfate | HMDB | | 3,5,3'-triiodo-L-Thyronine 4-O-sulphate | HMDB | | 3-[4-(4-Hydroxy-3-iodophenoxy-4-O-sulfate)-3,5-diiodophenyl]-L-alanine | HMDB | | 3-[4-(4-Hydroxy-3-iodophenoxy-4-O-sulphate)-3,5-diiodophenyl]-L-alanine | HMDB | | O-(4-Hydroxy-3-iodophenyl-4-O-sulfate)-3,5-diiodo-L-tyrosine | HMDB | | O-(4-Hydroxy-3-iodophenyl-4-O-sulphate)-3,5-diiodo-L-tyrosine | HMDB | | T3S | HMDB | | 3,3',5-Triiodo-L-thyronine sulfate | HMDB | | 3,5,3'-Triiodothyronine-4-sulfate | HMDB | | Triiodothyronine sulfate | ChEBI | | 3,3',5-Triiodo-L-thyronine sulfuric acid | Generator, HMDB | | 3,3',5-Triiodo-L-thyronine sulphate | Generator, HMDB | | 3,3',5-Triiodo-L-thyronine sulphuric acid | Generator, HMDB |
|
|---|
| Chemical Formula | C15H12I3NO7S |
|---|
| Average Molecular Weight | 731.037 |
|---|
| Monoisotopic Molecular Weight | 730.746852693 |
|---|
| IUPAC Name | (2S)-2-amino-3-{3,5-diiodo-4-[3-iodo-4-(sulfooxy)phenoxy]phenyl}propanoic acid |
|---|
| Traditional Name | triiodothyronine sulfate |
|---|
| CAS Registry Number | 31135-55-4 |
|---|
| SMILES | N[C@@H](CC1=CC(I)=C(OC2=CC(I)=C(OS(O)(=O)=O)C=C2)C(I)=C1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C15H12I3NO7S/c16-9-6-8(1-2-13(9)26-27(22,23)24)25-14-10(17)3-7(4-11(14)18)5-12(19)15(20)21/h1-4,6,12H,5,19H2,(H,20,21)(H,22,23,24)/t12-/m0/s1 |
|---|
| InChI Key | XBQYQXVJBNDCGY-LBPRGKRZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylalanine and derivatives. Phenylalanine and derivatives are compounds containing phenylalanine or a derivative thereof resulting from reaction of phenylalanine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Phenylalanine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylalanine or derivatives
- Diphenylether
- 3-phenylpropanoic-acid
- Phenylsulfate
- Diaryl ether
- Alpha-amino acid
- Amphetamine or derivatives
- L-alpha-amino acid
- Arylsulfate
- Phenoxy compound
- Phenol ether
- Halobenzene
- Aralkylamine
- Iodobenzene
- Monocyclic benzene moiety
- Benzenoid
- Sulfuric acid ester
- Sulfate-ester
- Sulfuric acid monoester
- Aryl halide
- Aryl iodide
- Organic sulfuric acid or derivatives
- Amino acid
- Carboxylic acid
- Ether
- Monocarboxylic acid or derivatives
- Primary aliphatic amine
- Organonitrogen compound
- Organooxygen compound
- Amine
- Primary amine
- Organic nitrogen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organohalogen compound
- Organoiodide
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-053l-5013009000-1c6c0500627c3200e1ed | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01q0-0001009700-2d0aab12c337820146f5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05n0-0000009100-b7da24544e575f9968e9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-016r-0019021000-07517127273a1b5bf2c2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0001002900-f22497117ff7d082125a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01rt-1012019200-f905c77e90631dea70e4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00e9-9032148000-75e93100ebb30ee89a89 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0000002900-09c194d1adffdfe3de89 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-053i-0000009200-d3859140cdd21f699479 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052r-0000079000-365a6ef91738cc1d24ae | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000000900-28dbe4926702ec142290 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-2900012500-24d71655d2f3349b1e2d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-0900000000-0dba40d354fa6a5575e0 | View in MoNA |
|---|
|
|---|