| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:03:18 UTC |
|---|
| Update Date | 2020-04-22 15:11:53 UTC |
|---|
| BMDB ID | BMDB0003240 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
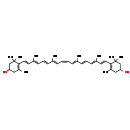
| Common Name | (3R,3'R,15-cis)-b,b-Carotene-3,3'-diol |
|---|
| Description | Zeaxanthin belongs to the class of organic compounds known as xanthophylls. These are carotenoids containing an oxygenated carotene backbone. Carotenes are characterized by the presence of two end-groups (mostly cyclohexene rings, but also cyclopentene rings or acyclic groups) linked by a long branched alkyl chain. Carotenes belonging form a subgroup of the carotenoids family. Xanthophylls arise by oxygenation of the carotene backbone. Zeaxanthin is possibly soluble (in water) and an extremely weak basic (essentially neutral) compound (based on its pKa). Zeaxanthin exists in all living organisms, ranging from bacteria to humans. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3R,3'r,15-cis)-b,b-Carotene-3,3'-diol | Generator | | (3R,3'r,15-cis)-Β,β-carotene-3,3'-diol | Generator | | (15Z)-Zeaxanthin | HMDB | | (3R,3'r,15-cis)-Zeaxanthin | HMDB | | (3R,3’R,15-cis)-zeaxanthin | HMDB | | (3R,3’R,15-cis)-β,β-carotene-3,3’-diol | HMDB | | 15-cis-Zeaxanthin | HMDB | | (3R,3'R,15-cis)-beta,beta-Carotene-3,3'-diol | HMDB |
|
|---|
| Chemical Formula | C40H56O2 |
|---|
| Average Molecular Weight | 568.886 |
|---|
| Monoisotopic Molecular Weight | 568.428031043 |
|---|
| IUPAC Name | (1R)-4-[(1E,3E,5E,7E,9Z,11E,13E,15E,17E)-18-[(4R)-4-hydroxy-2,6,6-trimethylcyclohex-1-en-1-yl]-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaen-1-yl]-3,5,5-trimethylcyclohex-3-en-1-ol |
|---|
| Traditional Name | 13Z-β,β-carotene-3R,3R'-diol |
|---|
| CAS Registry Number | 60046-54-0 |
|---|
| SMILES | C\C(\C=C\C=C(/C)\C=C\C1=C(C)C[C@@H](O)CC1(C)C)=C/C=C\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)C[C@@H](O)CC1(C)C |
|---|
| InChI Identifier | InChI=1S/C40H56O2/c1-29(17-13-19-31(3)21-23-37-33(5)25-35(41)27-39(37,7)8)15-11-12-16-30(2)18-14-20-32(4)22-24-38-34(6)26-36(42)28-40(38,9)10/h11-24,35-36,41-42H,25-28H2,1-10H3/b12-11-,17-13+,18-14+,23-21+,24-22+,29-15+,30-16+,31-19+,32-20+/t35-,36-/m1/s1 |
|---|
| InChI Key | JKQXZKUSFCKOGQ-RFVVUROPSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as xanthophylls. These are carotenoids containing an oxygenated carotene backbone. Carotenes are characterized by the presence of two end-groups (mostly cyclohexene rings, but also cyclopentene rings or acyclic groups) linked by a long branched alkyl chain. Carotenes belonging form a subgroup of the carotenoids family. Xanthophylls arise by oxygenation of the carotene backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Tetraterpenoids |
|---|
| Direct Parent | Xanthophylls |
|---|
| Alternative Parents | |
|---|
| Substituents | - Xanthophyll
- Secondary alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("(3R,3'R,15-cis)-beta,beta-Carotene-3,3'-diol,1TMS,#1" TMS) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0001090000-762864ffd0f72009c688 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0104290000-79f130816293d1629a5c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-003b-0229210000-8aa487c6a883739666a2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0132890000-50be05fa861115532d66 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ue9-0215950000-140f155070c122eab02c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0v00-0039600000-2725b4335e7d4d6e49c2 | View in MoNA |
|---|
|
|---|