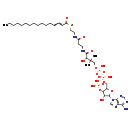
Showing metabocard for (2E)-Hexadecenoyl-CoA (BMDB0003945)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2016-09-30 23:06:54 UTC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2020-04-22 15:13:00 UTC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMDB ID | BMDB0003945 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Secondary Accession Numbers |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | (2E)-Hexadecenoyl-CoA | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | (2E)-Hexadecenoyl-CoA, also known as (e)-C16:1 N-14-CoA or trans-2-hexadecenoyl-CoA, belongs to the class of organic compounds known as long-chain 2-enoyl coas. These are organic compounds containing a coenzyme A substructure linked to a long-chain 2-enoyl chain of 13 to 21 carbon atoms. Based on a literature review very few articles have been published on (2E)-Hexadecenoyl-CoA. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C37H64N7O17P3S | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight | 1003.93 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight | 1003.329225797 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | {[5-(6-amino-9H-purin-9-yl)-2-[({[({3-[(2-{[2-(hexadec-2-enoylsulfanyl)ethyl]carbamoyl}ethyl)carbamoyl]-3-hydroxy-2,2-dimethylpropoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)methyl]-4-hydroxyoxolan-3-yl]oxy}phosphonic acid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | [5-(6-aminopurin-9-yl)-2-{[({3-[(2-{[2-(hexadec-2-enoylsulfanyl)ethyl]carbamoyl}ethyl)carbamoyl]-3-hydroxy-2,2-dimethylpropoxy(hydroxy)phosphoryl}oxy(hydroxy)phosphoryl)oxy]methyl}-4-hydroxyoxolan-3-yl]oxyphosphonic acid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 4460-95-1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | CCCCCCCCCCCCCC=CC(=O)SCCNC(=O)CCNC(=O)C(O)C(C)(C)COP(O)(=O)OP(O)(=O)OCC1OC(C(O)C1OP(O)(O)=O)N1C=NC2=C1N=CN=C2N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/C37H64N7O17P3S/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-17-28(46)65-21-20-39-27(45)18-19-40-35(49)32(48)37(2,3)23-58-64(55,56)61-63(53,54)57-22-26-31(60-62(50,51)52)30(47)36(59-26)44-25-43-29-33(38)41-24-42-34(29)44/h16-17,24-26,30-32,36,47-48H,4-15,18-23H2,1-3H3,(H,39,45)(H,40,49)(H,53,54)(H,55,56)(H2,38,41,42)(H2,50,51,52) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | JUPAQFRKPHPXLD-UHFFFAOYSA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as long-chain 2-enoyl coas. These are organic compounds containing a coenzyme A substructure linked to a long-chain 2-enoyl chain of 13 to 21 carbon atoms. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Lipids and lipid-like molecules | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Fatty Acyls | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Fatty acyl thioesters | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Long-chain 2-enoyl CoAs | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ontology | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Expected but not Quantified | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofunction | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Application | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular locations |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biospecimen Locations | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | HMDB0003945 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phenol Explorer Compound ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FooDB ID | FDB023264 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KNApSAcK ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemspider ID | 4292824 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG Compound ID | C05272 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BiGG ID | 45474 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| METLIN ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound | 5118010 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | 61526 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Al-Arif, Adhid; Blecher, Melvin. Chemical synthesis of carnitine and coenzyme A esters of the b-substituted intermediates of hexadecanoic acid metabolism. Biochimica et Biophysica Acta, Lipids and Lipid Metabolism (1971), 248(3), 416-29. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||