| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:07:42 UTC |
|---|
| Update Date | 2020-05-21 16:28:50 UTC |
|---|
| BMDB ID | BMDB0004077 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 4,6-Dihydroxyquinoline |
|---|
| Description | 4,6-Dihydroxyquinoline, also known as 4,6-dihydroxyquinoline, belongs to the class of organic compounds known as hydroxyquinolones. Hydroxyquinolones are compounds containing a quinoline moiety bearing a hydroxyl group and a ketone. Quinoline or benzo[b]pyridine is a bicyclic compound that consists of benzene fused to a pyridine. 4,6-Dihydroxyquinoline is possibly soluble (in water) and a moderately basic compound (based on its pKa). 4,6-Dihydroxyquinoline exists in all living organisms, ranging from bacteria to humans. 4,6-Dihydroxyquinoline can be biosynthesized from 5-hydroxykynurenamine through its interaction with the enzyme kynurenine 3-monooxygenase. In cattle, 4,6-dihydroxyquinoline is involved in the metabolic pathway called the tryptophan metabolism pathway. |
|---|
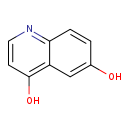
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 6-Hydroxy-1H-quinolin-4-one | ChEBI | | Quinoline-4,6-diol | Kegg | | 4,6-Quinolinediol | HMDB |
|
|---|
| Chemical Formula | C9H7NO2 |
|---|
| Average Molecular Weight | 161.1574 |
|---|
| Monoisotopic Molecular Weight | 161.047678473 |
|---|
| IUPAC Name | quinoline-4,6-diol |
|---|
| Traditional Name | 4,6-dihydroxyquinoline |
|---|
| CAS Registry Number | 3517-61-1 |
|---|
| SMILES | OC1=CC2=C(O)C=CN=C2C=C1 |
|---|
| InChI Identifier | InChI=1S/C9H7NO2/c11-6-1-2-8-7(5-6)9(12)3-4-10-8/h1-5,11H,(H,10,12) |
|---|
| InChI Key | XFALURCRIGINGT-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hydroxyquinolones. Hydroxyquinolones are compounds containing a quinoline moiety bearing a hydroxyl group and a ketone. Quinoline or benzo[b]pyridine is a bicyclic compound that consists of benzene fused to a pyridine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Quinolines and derivatives |
|---|
| Sub Class | Quinolones and derivatives |
|---|
| Direct Parent | Hydroxyquinolones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydroxyquinolone
- Dihydroquinolone
- Hydroxyquinoline
- Dihydroquinoline
- 1-hydroxy-2-unsubstituted benzenoid
- Pyridine
- Benzenoid
- Heteroaromatic compound
- Vinylogous amide
- Azacycle
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01q9-0900000000-5a26ca29602b82b947ec | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00ec-4190000000-4bb8d953c5850dd96f48 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0900000000-2b4e8673d9953857188d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0900000000-664352c4cf4241c0a1c4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-053r-2900000000-05e37aff72635356f2cc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0900000000-34a29a4deafcb0ad7c33 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0900000000-06fb975c74de0f90f723 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-053r-0900000000-39deb8142073ba5b68ae | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0900000000-d2b094746088a17847e1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0900000000-d9bf4aaec04c4b90185a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0159-4900000000-d7445a5c12b24666d75a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0900000000-29ea448bd0cee5fb98df | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0900000000-02e9863a91ed972b84eb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-053r-5900000000-f7f1ae1acee5ecfdcc35 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|
| Synthesis Reference | Noguchi, Tomoo; Kaseda, Hiroki; Kido, Ryo; Matsumura, Yuichi. 5-Hydroxykynurenine decarboxylase in rat intestine. Journal of Biochemistry (Tokyo, Japan) (1970), 67(1), 113-21. |
|---|