| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:09:12 UTC |
|---|
| Update Date | 2020-04-22 15:13:43 UTC |
|---|
| BMDB ID | BMDB0004321 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | (+)-Limonene |
|---|
| Description | D-Limonene, also known as L-limonen or dipentene, belongs to the class of organic compounds known as menthane monoterpenoids. These are monoterpenoids with a structure based on the o-, m-, or p-menthane backbone. P-menthane consists of the cyclohexane ring with a methyl group and a (2-methyl)-propyl group at the 1 and 4 ring position, respectively. The o- and m- menthanes are much rarer, and presumably arise by alkyl migration of p-menthanes. Thus, D-limonene is considered to be an isoprenoid lipid molecule. D-Limonene exists as a solid, very hydrophobic, practically insoluble (in water), and relatively neutral molecule. D-Limonene exists in all living organisms, ranging from bacteria to humans. |
|---|
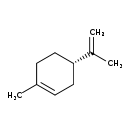
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (-)-(S)-Limonene | ChEBI | | (-)-(4S)-Limonene | ChEBI | | (4S)-1-Methyl-4-isopropenylcyclohex-1-ene | ChEBI | | (4S)-4-Isopropenyl-1-methylcyclohexene | ChEBI | | (S)-(-)-p-Mentha-1,8-diene | ChEBI | | (S)-1-Methyl-4-(1-methylethenyl)cyclohexene | ChEBI | | (S)-p-Mentha-1,8-diene | ChEBI | | 4AlphaH-p-mentha-1,8-diene | ChEBI | | L-Limonen | ChEBI | | L-Limonene | ChEBI | | 4-Isopropenyl-1-methyl-1-cyclohexene | HMDB | | Limonene | HMDB | | (+)-Limonene | HMDB | | AISA 5203-L (+)limonene | HMDB | | Dipentene | HMDB | | 1-Methyl-4-(1-methylethenyl)cyclohexene | HMDB | | Limonene, (+-)-isomer | HMDB | | (D)-Limonene | HMDB | | 4-Mentha-1,8-diene | HMDB | | Limonene, (S)-isomer | HMDB | | Limonene, (R)-isomer | HMDB | | (R)-(+)-Limonene | HMDB | | (R)-4-Isopropenyl-1-methylcyclohexene | HMDB | | (+)-(R)-4-Isopropenyl-1-methylcyclohexene | HMDB | | (4R)-1-Methyl-4-(1-methylethenyl)cyclohexene | HMDB | | D-Limonene | HMDB | | 4 Mentha 1,8 diene | HMDB | | D Limonene | HMDB | | (-)-alpha-Limonene | HMDB | | (-)-Α-limonene | HMDB | | (4S)-1-Methyl-4-(1-methylethenyl)cyclohexene | HMDB | | (4S)-Limonene | HMDB | | (S)-(-)-Limonene | HMDB | | (S)-1-Methyl-4-(prop-1-en-2-yl)cyclohex-1-ene | HMDB | | (S)-4-Isopropenyl-1-methyl-1-cyclohexene | HMDB | | (S)-Limonene | HMDB | | beta-Limonene | HMDB | | L-Carvene | HMDB | | Β-limonene | HMDB | | (-)-Limonene | ChEBI |
|
|---|
| Chemical Formula | C10H16 |
|---|
| Average Molecular Weight | 136.234 |
|---|
| Monoisotopic Molecular Weight | 136.125200512 |
|---|
| IUPAC Name | (4S)-1-methyl-4-(prop-1-en-2-yl)cyclohex-1-ene |
|---|
| Traditional Name | (-)-limonene |
|---|
| CAS Registry Number | 5989-27-5 |
|---|
| SMILES | CC(=C)[C@H]1CCC(C)=CC1 |
|---|
| InChI Identifier | InChI=1S/C10H16/c1-8(2)10-6-4-9(3)5-7-10/h4,10H,1,5-7H2,2-3H3/t10-/m1/s1 |
|---|
| InChI Key | XMGQYMWWDOXHJM-SNVBAGLBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as menthane monoterpenoids. These are monoterpenoids with a structure based on the o-, m-, or p-menthane backbone. P-menthane consists of the cyclohexane ring with a methyl group and a (2-methyl)-propyl group at the 1 and 4 ring position, respectively. The o- and m- menthanes are much rarer, and presumably arise by alkyl migration of p-menthanes. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Monoterpenoids |
|---|
| Direct Parent | Menthane monoterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - P-menthane monoterpenoid
- Monocyclic monoterpenoid
- Branched unsaturated hydrocarbon
- Cycloalkene
- Cyclic olefin
- Unsaturated aliphatic hydrocarbon
- Unsaturated hydrocarbon
- Olefin
- Hydrocarbon
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | -74.3 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 0.0138 mg/mL at 25 °C | Not Available | | LogP | 4.57 | LI,J & PURDUE,EM (1995) |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0173-9100000000-239928f205c8242a700a | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-014l-9100000000-507f7afbc17b7c2556e2 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-014l-9100000000-1a0c62c6971532ab6782 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0173-9100000000-239928f205c8242a700a | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-014l-9100000000-507f7afbc17b7c2556e2 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-014l-9100000000-1a0c62c6971532ab6782 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fgo-9200000000-a79c6ccef83c7113ab9f | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-001c-8900000000-dfd6c14e6a4e49bfc6dc | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-002f-9000000000-06c43b864c090aaae8ae | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-01t9-9000000000-e5ee54500bc5feda4631 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-1900000000-5567709cc9ec1c9909db | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-9600000000-4570df18c5140586d0bf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-1000-9100000000-61312f12f069cd9de75c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0900000000-88ada45edfe65d9e7f3a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0900000000-1192305465e6c746cfa9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kr-4900000000-199ee0c6ed49a35bcff4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001a-9400000000-fb09a1cdd4bc09480281 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0564-9000000000-dc5effa7f00987fc8567 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00mo-9000000000-40d99aa482e2ce5a66f1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0900000000-a013b4ae27f975ab5621 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0900000000-a013b4ae27f975ab5621 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-8900000000-d835e92fba2c2a238797 | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-014l-9100000000-7e8881e736cecb723a2b | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, CDCl3, experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, CDCl3, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|