| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:10:35 UTC |
|---|
| Update Date | 2020-04-22 15:14:08 UTC |
|---|
| BMDB ID | BMDB0004812 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 2,5-Furandicarboxylic acid |
|---|
| Description | 2,5-Furandicarboxylic acid, also known as 2,5-dicarboxyfuran or dehydromucic acid, belongs to the class of organic compounds known as furoic acids. These are organic compounds containing a furoic acid moiety, with a structure characterized by a furan ring bearing a carboxylic acid group at the C2 or C3 carbon atom. 2,5-Furandicarboxylic acid exists in all living organisms, ranging from bacteria to humans. Based on a literature review a small amount of articles have been published on 2,5-Furandicarboxylic acid. |
|---|
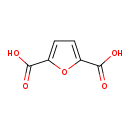
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,5-Dicarboxyfuran | ChEBI | | Dehydromucic acid | ChEBI | | Furane-alpha,alpha'-dicarboxylic acid | ChEBI | | Furan-2,5-dicarboxylate | Kegg | | Dehydromucate | Generator | | Furane-a,alpha'-dicarboxylate | Generator | | Furane-a,alpha'-dicarboxylic acid | Generator | | Furane-alpha,alpha'-dicarboxylate | Generator | | Furane-α,alpha'-dicarboxylate | Generator | | Furane-α,alpha'-dicarboxylic acid | Generator | | Furan-2,5-dicarboxylic acid | Generator | | 2,5-Furandicarboxylate | Generator | | (1,5-Dimethylhexyl)hydrazine | HMDB | | Dehydroschleimsaeure | HMDB | | Furan 2,5-dicarboxylate | HMDB | | Furan 2,5-dicarboxylic acid | HMDB | | Furan-2,5-dicarbonsaeure | HMDB | | Furane-a,a'-dicarboxylate | HMDB | | Furane-a,a'-dicarboxylic acid | HMDB | | FDCA CPD | MeSH, HMDB | | 2,5-Furandicarboxylic acid | ChEBI |
|
|---|
| Chemical Formula | C6H4O5 |
|---|
| Average Molecular Weight | 156.093 |
|---|
| Monoisotopic Molecular Weight | 156.005873238 |
|---|
| IUPAC Name | furan-2,5-dicarboxylic acid |
|---|
| Traditional Name | 2,5-furandicarboxylic acid |
|---|
| CAS Registry Number | 3238-40-2 |
|---|
| SMILES | OC(=O)C1=CC=C(O1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C6H4O5/c7-5(8)3-1-2-4(11-3)6(9)10/h1-2H,(H,7,8)(H,9,10) |
|---|
| InChI Key | CHTHALBTIRVDBM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as furoic acids. These are organic compounds containing a furoic acid moiety, with a structure characterized by a furan ring bearing a carboxylic acid group at the C2 or C3 carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Furans |
|---|
| Sub Class | Furoic acid and derivatives |
|---|
| Direct Parent | Furoic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Furoic acid
- Dicarboxylic acid or derivatives
- Heteroaromatic compound
- Oxacycle
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 342 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 1 mg/mL at 18 °C | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a5i-6900000000-d288769ec5810d63b1b2 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0229-9480000000-8391a736007498218310 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-000i-0900000000-2edf2743af7ee90a29ae | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-000l-9200000000-a9709e652d30a2063847 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-00ku-9000000000-c9fcb98abb314bb78c67 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-0900000000-c2840f2e7256d798d4fa | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052r-0900000000-c3d20c4a56763ab0900c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000j-9100000000-0e380e427de8c76266bf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-ec73c307d1a6f5bd94d2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0w29-2900000000-0b5e65af058aa2ef6db6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00lr-9200000000-41c982464657e52513de | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-02t9-9500000000-ea0c20378ff74ce1345f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-9100000000-4520e50e1c56f6782681 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-9000000000-eb1155a9bb1577d1bb89 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-1900000000-9fe216ad8db30c7901bb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-9600000000-bfeb15a5dbae92e0bbcd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-9000000000-524d51557840cf7b7f3c | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, Methanol, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, Methanol, experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, CD3OD, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|