| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:18:55 UTC |
|---|
| Update Date | 2020-04-22 15:16:26 UTC |
|---|
| BMDB ID | BMDB0005817 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 2-Acetamido-2-deoxy-6-O-a-L-fucopyranosyl-D-glucose |
|---|
| Description | 2-Acetamido-2-deoxy-6-O-a-L-fucopyranosyl-D-glucose belongs to the class of organic compounds known as fatty acyl glycosides of mono- and disaccharides. Fatty acyl glycosides of mono- and disaccharides are compounds composed of a mono- or disaccharide moiety linked to one hydroxyl group of a fatty alcohol or of a phosphorylated alcohol (phosphoprenols), a hydroxy fatty acid or to one carboxyl group of a fatty acid (ester linkage) or to an amino alcohol. Based on a literature review very few articles have been published on 2-Acetamido-2-deoxy-6-O-a-L-fucopyranosyl-D-glucose. |
|---|
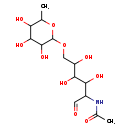
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-(acetylamino)-2-Deoxy-6-O-(6-deoxy-a-L-galactopyranosyl)-D-glucose | HMDB | | 2-(acetylamino)-2-Deoxy-6-O-(6-deoxy-alpha-L-galactopyranosyl)-D-glucose | HMDB | | 2-(acetylamino)-2-Deoxy-6-O-(6-deoxy-alpha-L-galactopyranosyl)-delta-glucose | HMDB | | N-{3,4,5-trihydroxy-1-oxo-6-[(3,4,5-trihydroxy-6-methyloxan-2-yl)oxy]hexan-2-yl}ethanimidate | Generator, HMDB | | 2-Acetamido-2-deoxy-6-O-a-L-fucopyranosyl-D-glucose | Generator | | 2-Acetamido-2-deoxy-6-O-α-L-fucopyranosyl-D-glucose | Generator |
|
|---|
| Chemical Formula | C14H25NO10 |
|---|
| Average Molecular Weight | 367.349 |
|---|
| Monoisotopic Molecular Weight | 367.147846025 |
|---|
| IUPAC Name | N-{3,4,5-trihydroxy-1-oxo-6-[(3,4,5-trihydroxy-6-methyloxan-2-yl)oxy]hexan-2-yl}acetamide |
|---|
| Traditional Name | N-{3,4,5-trihydroxy-1-oxo-6-[(3,4,5-trihydroxy-6-methyloxan-2-yl)oxy]hexan-2-yl}acetamide |
|---|
| CAS Registry Number | 37776-59-3 |
|---|
| SMILES | CC1OC(OCC(O)C(O)C(O)C(NC(C)=O)C=O)C(O)C(O)C1O |
|---|
| InChI Identifier | InChI=1S/C14H25NO10/c1-5-9(19)12(22)13(23)14(25-5)24-4-8(18)11(21)10(20)7(3-16)15-6(2)17/h3,5,7-14,18-23H,4H2,1-2H3,(H,15,17) |
|---|
| InChI Key | YBWAUUBLHOFOPK-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as fatty acyl glycosides of mono- and disaccharides. Fatty acyl glycosides of mono- and disaccharides are compounds composed of a mono- or disaccharide moiety linked to one hydroxyl group of a fatty alcohol or of a phosphorylated alcohol (phosphoprenols), a hydroxy fatty acid or to one carboxyl group of a fatty acid (ester linkage) or to an amino alcohol. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acyl glycosides |
|---|
| Direct Parent | Fatty acyl glycosides of mono- and disaccharides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Fatty acyl glycoside of mono- or disaccharide
- Alkyl glycoside
- Hexose monosaccharide
- Glycosyl compound
- O-glycosyl compound
- Amino saccharide
- Beta-hydroxy aldehyde
- Monosaccharide
- Oxane
- Acetamide
- Carboxamide group
- Secondary carboxylic acid amide
- Secondary alcohol
- Acetal
- Carboxylic acid derivative
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Hydrocarbon derivative
- Organic oxide
- Alcohol
- Organopnictogen compound
- Carbonyl group
- Organic oxygen compound
- Aldehyde
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0lxt-8977000000-a2bb860c76008c8ba468 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0006-4236119000-157bdb7a4e39cf80488e | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uxr-1219000000-f15747e1b5ccf9e81e65 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0pb9-9720000000-19137fd08ea152aadd6d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0w29-8900000000-f71c7291db908ebd248e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0cea-1923000000-caffbe52ec84c868cb4d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052b-4921000000-71facf6e0f221fb8e3d4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9710000000-c1d713140c972dfce842 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0229000000-899d186fea014b0d7629 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0kh9-9411000000-2a9b99c9e48ef6125eb2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ac0-9320000000-466625b9719e971e162a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-9315000000-e449249c04bae5502537 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9300000000-494e7c53dfe677c9151e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9100000000-e1289f85de752e1143d5 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|