| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:20:23 UTC |
|---|
| Update Date | 2020-04-22 15:16:55 UTC |
|---|
| BMDB ID | BMDB0006213 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
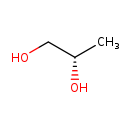
| Common Name | (S)-Propane-1,2-diol |
|---|
| Description | (S)-Propane-1,2-diol, also known as (S)-1,2-propanediol or (S)-propylene glycol, belongs to the class of organic compounds known as 1,2-diols. These are polyols containing an alcohol group at two adjacent positions (S)-Propane-1,2-diol exists in all living organisms, ranging from bacteria to humans. Based on a literature review a significant number of articles have been published on (S)-Propane-1,2-diol. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (S)-1,2-Propanediol | ChEBI | | (S)-Propylene glycol | ChEBI | | (2S)-Propane-1,2-diol | HMDB | | (+)-(S)-1,2-Propanediol | HMDB | | (+)-1,2-Propanediol | HMDB | | (2S)-1,2-Propanediol | HMDB | | (S)-(+)-Propane-1,2-diol | HMDB | | (S)-(+)-Propylene glycol | HMDB | | (S)-2-Hydroxy-1-propanol | HMDB | | (S)-2-Hydroxypropanol | HMDB | | (S)-Propane-1,2-diol | HMDB | | 1,2(S)-Propanediol | HMDB | | 1,2-(RS)-Propanediol | HMDB | | 1,2-Dihydroxypropane | HMDB | | 1,2-Propanediol | HMDB | | 1,2-Propylene glycol | HMDB | | 2,3-Propanediol | HMDB | | 2-Hydroxypropanol | HMDB | | 3-Deoxy-sn-glycerol | HMDB | | Isopropylene glycol | HMDB | | L-(+)-Propanediol | HMDB | | L-(+)-Propylene glycol | HMDB | | L-1,2-Propanediol | HMDB | | Methylethyl glycol | HMDB | | Methylethylene glycol | HMDB | | Monopropylene glycol | HMDB | | Propylene glycol | HMDB | | alpha-Propylene glycol | HMDB | | α-Propylene glycol | HMDB |
|
|---|
| Chemical Formula | C3H8O2 |
|---|
| Average Molecular Weight | 76.0944 |
|---|
| Monoisotopic Molecular Weight | 76.0524295 |
|---|
| IUPAC Name | (2S)-propane-1,2-diol |
|---|
| Traditional Name | (S)-1,2-propanediol |
|---|
| CAS Registry Number | 4254-15-3 |
|---|
| SMILES | C[C@H](O)CO |
|---|
| InChI Identifier | InChI=1S/C3H8O2/c1-3(5)2-4/h3-5H,2H2,1H3/t3-/m0/s1 |
|---|
| InChI Key | DNIAPMSPPWPWGF-VKHMYHEASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,2-diols. These are polyols containing an alcohol group at two adjacent positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Alcohols and polyols |
|---|
| Direct Parent | 1,2-diols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Secondary alcohol
- 1,2-diol
- Hydrocarbon derivative
- Primary alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-055g-9000000000-27df50480b6cca9d0e9b | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0kp0-9520000000-ffe4848ba88742d1a4d6 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-9000000000-0a1a58ae50ca7bfbc92f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6r-9000000000-4f58eaf424aa13185ca2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9000000000-d0e5def95a7a7879dc71 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-9000000000-5bf5d9d3c7cf604af9a3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056r-9000000000-ea5b27ac0cc585591f63 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000000-ecc48f7be3304c6cc1e0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a6r-9000000000-ea4d9d80ebf7615e6811 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9000000000-a77309b9b0e17e94870d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-7bb23415e61ec73a459c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4l-9000000000-5dba4634f494296e1347 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4l-9000000000-2174cc6a153316ad8c52 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-a1a685f935041163641b | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|