| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:22:16 UTC |
|---|
| Update Date | 2020-04-22 15:17:31 UTC |
|---|
| BMDB ID | BMDB0006468 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
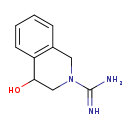
| Common Name | 4-Hydroxydebrisoquine |
|---|
| Description | 4-Hydroxydebrisoquine belongs to the class of organic compounds known as tetrahydroisoquinolines. These are tetrahydrogenated isoquinoline derivatives. Based on a literature review a significant number of articles have been published on 4-Hydroxydebrisoquine. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,4-Dihydro-4-hydroxy-2(1H)-isoquinolinecarboximidamide | ChEBI | | 4 Hydroxy debrisoquine | HMDB | | 4-Hydroxydebrisoquin sulfate (2:1) | MeSH, HMDB | | 4-Hydroxydebrisoquin | MeSH, HMDB | | 4-Hydroxydebrisoquine | MeSH |
|
|---|
| Chemical Formula | C10H13N3O |
|---|
| Average Molecular Weight | 191.2297 |
|---|
| Monoisotopic Molecular Weight | 191.105862053 |
|---|
| IUPAC Name | 4-hydroxy-1,2,3,4-tetrahydroisoquinoline-2-carboximidamide |
|---|
| Traditional Name | 4-hydroxydebrisoquine |
|---|
| CAS Registry Number | 59333-79-8 |
|---|
| SMILES | NC(=N)N1CC(O)C2=C(C1)C=CC=C2 |
|---|
| InChI Identifier | InChI=1S/C10H13N3O/c11-10(12)13-5-7-3-1-2-4-8(7)9(14)6-13/h1-4,9,14H,5-6H2,(H3,11,12) |
|---|
| InChI Key | AKFURXZANOMQBD-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tetrahydroisoquinolines. These are tetrahydrogenated isoquinoline derivatives. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrahydroisoquinolines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Tetrahydroisoquinolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tetrahydroisoquinoline
- Benzenoid
- Secondary alcohol
- Guanidine
- Azacycle
- Carboximidamide
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Imine
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-006x-2900000000-65d9860661c52fc43fed | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00di-7930000000-6c547ba47e9a793be64a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dl-0900000000-d27d767387884b5344ee | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0900000000-b4e6a4b85e95d2cd1a4d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0kfx-9700000000-746c466d9e27ab653e6a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0007-0900000000-4a585452b243c3fa4da6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001j-0900000000-b7230e5ec4140e46052d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-0a94add8e365b703b0d3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-006x-0900000000-3e730cd5607d9222d8e2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000t-0900000000-457a2f2c96a4c0185715 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-1900000000-65c31420b1e8b7d91d1c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0900000000-b4234df44cdc9ae7eb0d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0900000000-c646f107e3db6aef76a2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000x-6900000000-6b6670295b89ecb7441a | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|
| Synthesis Reference | Ram, Siya; Saxena, Anil K.; Jain, Padam C. 2-Substituted 4-hydroxy-1,2,3,4-tetrahydroisoquinolines. Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry (1978), 16B(11), 1019-22. |
|---|