| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:25:05 UTC |
|---|
| Update Date | 2020-04-22 15:18:18 UTC |
|---|
| BMDB ID | BMDB0006709 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Ubiquinone Q2 |
|---|
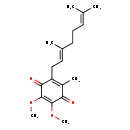
| Description | Ubiquinone-2, also known as ubiquinone 10 or coenzyme Q, belongs to the class of organic compounds known as ubiquinones. These are coenzyme Q derivatives containing a 5, 6-dimethoxy-3-methyl(1,4-benzoquinone) moiety to which an isoprenyl group is attached at ring position 2(or 6). Based on a literature review a significant number of articles have been published on Ubiquinone-2. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (e)-2-(3,7-Dimethyl-2,6-octadienyl)-5,6-dimethoxy-3-methyl-p-benzoquinone | ChEBI | | 2-[(2E)-3,7-Dimethylocta-2,6-dien-1-yl]-5,6-dimethoxy-3-methyl-1,4-benzoquinone | ChEBI | | Ubiquinone 10 | ChEBI | | Coenzyme Q | Kegg | | CoQ | Kegg | | Q | Kegg | | 2,3-Dimethoxy-5-geranyl-6-methyl-1,4-benzoquinone | HMDB | | 2-(3,7-Dimethyl-2,6-octadienyl)-5,6-dimethoxy-3-methyl- P-benzoquinone | HMDB | | 2-(3,7-Dimethyl-2,6-octadienyl)-5,6-dimethoxy-3-methyl-2,5-cyclohexadiene-1,4-dione | HMDB | | 2-[(2E)-3,7-Dimethyl-2,6-octadienyl]-5,6-dimethoxy-3-methyl- P-benzoquinone | HMDB | | Coenzyme Q2 | HMDB | | Q 2 | HMDB | | Ubiquinone 2 | HMDB | | Ubiquinone Q2 | HMDB | | Ubiquinone Q2, (e)-isomer | MeSH, HMDB |
|
|---|
| Chemical Formula | C19H26O4 |
|---|
| Average Molecular Weight | 318.4073 |
|---|
| Monoisotopic Molecular Weight | 318.18310932 |
|---|
| IUPAC Name | 2-[(2E)-3,7-dimethylocta-2,6-dien-1-yl]-5,6-dimethoxy-3-methylcyclohexa-2,5-diene-1,4-dione |
|---|
| Traditional Name | coenzyme-Q |
|---|
| CAS Registry Number | 606-06-4 |
|---|
| SMILES | COC1=C(OC)C(=O)C(C\C=C(/C)CCC=C(C)C)=C(C)C1=O |
|---|
| InChI Identifier | InChI=1S/C19H26O4/c1-12(2)8-7-9-13(3)10-11-15-14(4)16(20)18(22-5)19(23-6)17(15)21/h8,10H,7,9,11H2,1-6H3/b13-10+ |
|---|
| InChI Key | SQQWBSBBCSFQGC-JLHYYAGUSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as ubiquinones. These are coenzyme Q derivatives containing a 5, 6-dimethoxy-3-methyl(1,4-benzoquinone) moiety to which an isoprenyl group is attached at ring position 2(or 6). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Quinone and hydroquinone lipids |
|---|
| Direct Parent | Ubiquinones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Ubiquinone skeleton
- Monoterpenoid
- Monocyclic monoterpenoid
- Quinone
- P-benzoquinone
- Vinylogous ester
- Cyclic ketone
- Ketone
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udi-4394000000-4392d038cb40d1c25736 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-0fnj-2940000000-9de99f5e60d85c76bc22 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-00kb-0694000000-0e7666219469e4d3daa4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0109000000-46935a3400936bee8dab | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-00kb-0694000000-1aa3da1d9a8adf74884c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0439000000-180eeec0662a1cd541e8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01ba-3942000000-6814d10b6c7404b88af9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-9410000000-95413a2c0ab148a85d9e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0019000000-f5070dbbdd24c4bc12aa | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-02t9-0296000000-7c0eaf226e5a4866b999 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-9361000000-fff86254a78962e3b850 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-9c9a5615e70cdf418e9b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014r-0696000000-31579df78d92b1e7acbf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0v7r-0491000000-cc67df533d00a14ce722 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014j-0739000000-503861a7af6bafcfa25f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-3951000000-41b9dda5990696c36275 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a5c-9540000000-b49cb12d9c6d12564ffd | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|