| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:25:10 UTC |
|---|
| Update Date | 2020-04-22 15:18:20 UTC |
|---|
| BMDB ID | BMDB0006721 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
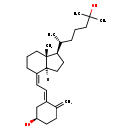
| Common Name | 5,6-trans-25-Hydroxyvitamin D3 |
|---|
| Description | 5,6-trans-25-Hydroxyvitamin D3, also known as 25-hydroxy-5,6-trans-vitamin D3 or 5,6-trans-25-hydroxycholecalciferol, belongs to the class of organic compounds known as vitamin d and derivatives. Vitamin D and derivatives are compounds containing a secosteroid backbone, usually secoergostane or secocholestane. Based on a literature review a small amount of articles have been published on 5,6-trans-25-Hydroxyvitamin D3. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3b,5E,7E)-9,10-Secocholesta-5,7,10(19)-triene-3,25-diol | HMDB | | (3beta,5E,7E)-9,10-Secocholesta-5,7,10(19)-triene-3,25-diol | HMDB | | 25-Hydroxy-5,6-trans-cholecalciferol | HMDB | | 25-Hydroxy-5,6-trans-vitamin D3 | HMDB | | 5,6-trans-25-Hydroxycholecalciferol | HMDB |
|
|---|
| Chemical Formula | C27H44O2 |
|---|
| Average Molecular Weight | 400.6371 |
|---|
| Monoisotopic Molecular Weight | 400.334130652 |
|---|
| IUPAC Name | (1R,3E)-3-{2-[(1R,4Z,7aR)-1-[(2R)-6-hydroxy-6-methylheptan-2-yl]-7a-methyl-octahydro-1H-inden-4-ylidene]ethylidene}-4-methylidenecyclohexan-1-ol |
|---|
| Traditional Name | 5,6-trans-25-hydroxyvitamin D3 |
|---|
| CAS Registry Number | 36149-00-5 |
|---|
| SMILES | C[C@H](CCCC(O)(C)C)[C@H]1CCC2([H])\C(CCC[C@]12C)=C/C=C1\C[C@H](O)CCC1=C |
|---|
| InChI Identifier | InChI=1S/C27H44O2/c1-19-10-13-23(28)18-22(19)12-11-21-9-7-17-27(5)24(14-15-25(21)27)20(2)8-6-16-26(3,4)29/h11-12,20,23-25,28-29H,1,6-10,13-18H2,2-5H3/b21-11-,22-12+/t20-,23-,24-,25?,27-/m1/s1 |
|---|
| InChI Key | JWUBBDSIWDLEOM-ZMHTYULMSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as vitamin d and derivatives. Vitamin D and derivatives are compounds containing a secosteroid backbone, usually secoergostane or secocholestane. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Vitamin D and derivatives |
|---|
| Direct Parent | Vitamin D and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Triterpenoid
- Tertiary alcohol
- Cyclic alcohol
- Secondary alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0ab9-3029000000-4c75b5cfbc422ba2a344 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-003r-1403290000-a8eb9294a6df1ce864d2 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00lr-0119100000-c93e02f4cad435f61273 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0aw9-1369000000-cbcc10bc94de9ec9da1b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05ai-5297000000-3c05eab14ebbc9c770e8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-cc66843e9af0092a8e53 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000t-0009000000-3efda332aebefe04f7ed | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00si-1129000000-ee8ae09159fb7ba45063 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0549100000-7e0d15fb33d297cd7b9d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0g4i-5494100000-ac59f398e11b390f5b2d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ab9-1940000000-65e563c113f344b0c1b6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-6962dcfe00557f780ffe | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0109000000-479fbd2eb75d5147bbc7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01pk-0339000000-a9adae13919854810a00 | View in MoNA |
|---|
|
|---|
| Synthesis Reference | Andrews, David R.; Barton, Derek H. R.; Hesse, Robert H.; Pechet, Maurice M. Synthesis of 25-hydroxy- and 1a,25-dihydroxy vitamin D3 from vitamin D2 (calciferol). Journal of Organic Chemistry (1986), 51(25), 4819-28. |
|---|