| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:26:26 UTC |
|---|
| Update Date | 2020-05-21 16:29:04 UTC |
|---|
| BMDB ID | BMDB0006823 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
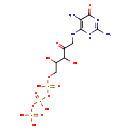
| Common Name | 2,5-Diamino-6-(5'-triphosphoryl-3',4'-trihydroxy-2'-oxopentyl)-amino-4-oxopyrimidine |
|---|
| Description | 2,5-Diamino-6-(5'-triphosphoryl-3',4'-trihydroxy-2'-oxopentyl)-amino-4-oxopyrimidine belongs to the class of organic compounds known as pentose phosphates. These are carbohydrate derivatives containing a pentose substituted by one or more phosphate groups. 2,5-Diamino-6-(5'-triphosphoryl-3',4'-trihydroxy-2'-oxopentyl)-amino-4-oxopyrimidine exists in all living organisms, ranging from bacteria to humans. Based on a literature review very few articles have been published on 2,5-Diamino-6-(5'-triphosphoryl-3',4'-trihydroxy-2'-oxopentyl)-amino-4-oxopyrimidine. |
|---|
| Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C9H18N5O14P3 |
|---|
| Average Molecular Weight | 513.1856 |
|---|
| Monoisotopic Molecular Weight | 513.006309845 |
|---|
| IUPAC Name | [({[({5-[(2,5-diamino-6-oxo-3,6-dihydropyrimidin-4-yl)amino]-2,3-dihydroxy-4-oxopentyl}oxy)(hydroxy)phosphoryl]oxy}(hydroxy)phosphoryl)oxy]phosphonic acid |
|---|
| Traditional Name | [({5-[(2,5-diamino-6-oxo-3H-pyrimidin-4-yl)amino]-2,3-dihydroxy-4-oxopentyl}oxy(hydroxy)phosphoryl)oxy(hydroxy)phosphoryl]oxyphosphonic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | NC1=NC(=O)C(N)=C(NCC(=O)C(O)C(O)COP(O)(=O)OP(O)(=O)OP(O)(O)=O)N1 |
|---|
| InChI Identifier | InChI=1S/C9H18N5O14P3/c10-5-7(13-9(11)14-8(5)18)12-1-3(15)6(17)4(16)2-26-30(22,23)28-31(24,25)27-29(19,20)21/h4,6,16-17H,1-2,10H2,(H,22,23)(H,24,25)(H2,19,20,21)(H4,11,12,13,14,18) |
|---|
| InChI Key | ZJYBJXKSWQPKFW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pentose phosphates. These are carbohydrate derivatives containing a pentose substituted by one or more phosphate groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Pentose phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pentose-5-phosphate
- Monosaccharide phosphate
- Pyrimidone
- Secondary aliphatic/aromatic amine
- Aminopyrimidine
- Monoalkyl phosphate
- Hydropyrimidine
- Phosphoric acid ester
- Beta-hydroxy ketone
- Pyrimidine
- Acyloin
- Organic phosphoric acid derivative
- Alkyl phosphate
- Alpha-hydroxy ketone
- Heteroaromatic compound
- Vinylogous amide
- 1,2-diol
- Secondary alcohol
- Ketone
- Secondary amine
- Organoheterocyclic compound
- Azacycle
- Organic nitrogen compound
- Organonitrogen compound
- Carbonyl group
- Primary amine
- Hydrocarbon derivative
- Amine
- Organic oxide
- Alcohol
- Organopnictogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0kaj-3695200000-3a55935f0cd1e0f2f9ef | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-004i-2194102000-c52d41bf51dcab1f48f4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01ot-1442920000-fe5a369793012336651c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0w2c-1941200000-110e7623a57abfccc156 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udj-5910000000-57cceddb7b071bdbb766 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0492220000-7b3e1f682e2fbbb3c9a5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-057l-9560000000-056e90117fa787c6136e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-056r-9620000000-81675245a86543331ea4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000090000-0e4a8635d042911dfe6b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-06vi-2510910000-88994d38e4fc655e8499 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9200000000-15a166740d6f7d46bd0f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000190000-3bbaea893a6dbc5b8ef5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0r0a-1148930000-ef0df46fe1647dbbfe7c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000f-1943000000-1ee8cf91c2cd1830fc9b | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|