| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:27:05 UTC |
|---|
| Update Date | 2020-05-21 16:28:29 UTC |
|---|
| BMDB ID | BMDB0006875 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
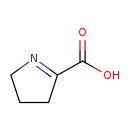
| Common Name | 1-Pyrroline-2-carboxylic acid |
|---|
| Description | 1-Pyrroline-2-carboxylic acid, also known as delta1-pyrroline 2-carboxylate, belongs to the class of organic compounds known as pyrrolines. Pyrrolines are compounds containing a pyrroline ring, which is a five-member unsaturated aliphatic ring with one nitrogen atom and four carbon atoms. 1-Pyrroline-2-carboxylic acid is a moderately basic compound (based on its pKa). 1-Pyrroline-2-carboxylic acid exists in all living organisms, ranging from bacteria to humans. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Pyrroline-2-carboxylate | ChEBI | | delta1-Pyrroline 2-carboxylate | ChEBI | | 1-Pyrroline 2-carboxylate | Kegg | | delta1-Pyrroline 2-carboxylic acid | Generator | | Δ1-pyrroline 2-carboxylate | Generator | | Δ1-pyrroline 2-carboxylic acid | Generator | | 1-Pyrroline 2-carboxylic acid | Generator | | 3,4-Dihydro-2H-pyrrole-5-carboxylate | HMDB | | 3,4-Dihydro-2H-pyrrole-5-carboxylic acid | HMDB | | D1-Pyrroline 2-carboxylate | HMDB | | D1-Pyrroline 2-carboxylic acid | HMDB | | delta-1-Pyrroline-2-carboxylate | HMDB | | 1,2-Didehydroproline | HMDB | | 1-Pyrroline-2-carboxylic acid | HMDB | | delta1-Pyrroline-2-carboxylic acid | HMDB | | Δ1-Pyrroline-2-carboxylic acid | HMDB |
|
|---|
| Chemical Formula | C5H7NO2 |
|---|
| Average Molecular Weight | 113.1146 |
|---|
| Monoisotopic Molecular Weight | 113.047678473 |
|---|
| IUPAC Name | 3,4-dihydro-2H-pyrrol-1-ium-5-carboxylate |
|---|
| Traditional Name | 4,5-dihydro-3H-pyrrol-1-ium-2-carboxylate |
|---|
| CAS Registry Number | 2139-03-9 |
|---|
| SMILES | OC(=O)C1=NCCC1 |
|---|
| InChI Identifier | InChI=1S/C5H7NO2/c7-5(8)4-2-1-3-6-4/h1-3H2,(H,7,8) |
|---|
| InChI Key | RHTAIKJZSXNELN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyrrolines. Pyrrolines are compounds containing a pyrroline ring, which is a five-member unsaturated aliphatic ring with one nitrogen atom and four carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyrrolines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Pyrrolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrroline
- Ketimine
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Azacycle
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carbonyl group
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Imine
- Organic oxygen compound
- Organic nitrogen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 TMS) | splash10-00di-2900000000-66ac333a416ee8f7a478 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-00di-2900000000-66ac333a416ee8f7a478 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00mo-9000000000-a7ad471aae5db0467dfa | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03dj-9700000000-06b3e3d5411a8f705758 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-9100000000-8fa6f17fa32d2dc2a376 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-c48f7e9292b52e3d7a54 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-5900000000-b4814a13bcbaa249948d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-02t9-9400000000-d7552022e5be14c1afea | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014l-9000000000-c850159936b776efff87 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-9300000000-e23eb312ac037d064514 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-9000000000-4b6f8dab3c747051784c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-9000000000-43454e9f111a08bb0994 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-3900000000-8779645df27f611a2273 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-9200000000-e49e646efe87cb2ac8f1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kf-9000000000-b0c27aa3a5a4bfd16ebf | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|