| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:27:46 UTC |
|---|
| Update Date | 2020-04-22 15:19:07 UTC |
|---|
| BMDB ID | BMDB0006954 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
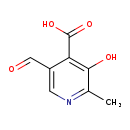
| Common Name | 2-Methyl-3-hydroxy-5-formylpyridine-4-carboxylate |
|---|
| Description | 2-Methyl-3-hydroxy-5-formylpyridine-4-carboxylate, also known as 2-methyl-3-hydroxy-5-formylpyridine-4-carboxylate, belongs to the class of organic compounds known as pyridinecarboxylic acids. Pyridinecarboxylic acids are compounds containing a pyridine ring bearing a carboxylic acid group. 2-Methyl-3-hydroxy-5-formylpyridine-4-carboxylate is possibly soluble (in water) and a moderately basic compound (based on its pKa). In cattle, 2-methyl-3-hydroxy-5-formylpyridine-4-carboxylate is involved in the metabolic pathway called the vitamin B6 metabolism pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Methyl-3-hydroxy-5-formylpyridine-4-carboxylic acid | ChEBI | | 5-Formyl-3-hydroxy-2-methyl-4-pyridinecarboxylic acid | ChEBI | | 5-Formyl-3-hydroxy-2-methylisonicotinic acid | ChEBI | | 5-Formyl-3-hydroxy-2-methylpyridine-4-carboxylate | Kegg | | 5-Formyl-3-hydroxy-2-methyl-4-pyridinecarboxylate | Generator | | 5-Formyl-3-hydroxy-2-methylisonicotinate | Generator | | 5-Formyl-3-hydroxy-2-methylpyridine-4-carboxylic acid | Generator |
|
|---|
| Chemical Formula | C8H7NO4 |
|---|
| Average Molecular Weight | 181.1455 |
|---|
| Monoisotopic Molecular Weight | 181.037507717 |
|---|
| IUPAC Name | 5-formyl-3-hydroxy-2-methylpyridine-4-carboxylic acid |
|---|
| Traditional Name | 5-formyl-3-hydroxy-2-methylpyridine-4-carboxylic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CC1=NC=C(C=O)C(C(O)=O)=C1O |
|---|
| InChI Identifier | InChI=1S/C8H7NO4/c1-4-7(11)6(8(12)13)5(3-10)2-9-4/h2-3,11H,1H3,(H,12,13) |
|---|
| InChI Key | JTWNWNJMNSJYDL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyridinecarboxylic acids. Pyridinecarboxylic acids are compounds containing a pyridine ring bearing a carboxylic acid group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyridines and derivatives |
|---|
| Sub Class | Pyridinecarboxylic acids and derivatives |
|---|
| Direct Parent | Pyridinecarboxylic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyridine carboxylic acid
- 3-pyridine carboxaldehyde
- Aryl-aldehyde
- Hydroxypyridine
- Methylpyridine
- Vinylogous acid
- Heteroaromatic compound
- Azacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Aldehyde
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0gwr-1900000000-3ef31f64e6cabae0db9a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0h99-9185000000-d05ebb431811e003661d | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01q9-0900000000-41fb79905547bb1d58b7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dr-0900000000-f58de110251e801c88b7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pb9-8900000000-f03a56176eaddbb5cc21 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001r-0900000000-f5192220502d8a33d310 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052r-0900000000-2f52718a323b73a125d7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9700000000-caefdcb51b7b757425e2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0900000000-7f7e2435fc582dfcfad0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4r-0900000000-ba78185c583c93e37222 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-6900000000-0c1e2d2a26b160b7591d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0900000000-3967d9650db5366bcf85 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dr-0900000000-2a6bb3953b415e0fa38d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052b-9600000000-97846af5421fbd89bac6 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|