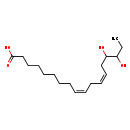| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:01:26 UTC |
|---|
| Update Date | 2020-04-22 15:39:26 UTC |
|---|
| BMDB ID | BMDB0010208 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 15,16-DiHODE |
|---|
| Description | 15,16-DiHODE, also known as a-15,16-dihode, belongs to the class of organic compounds known as lineolic acids and derivatives. These are derivatives of lineolic acid. Lineolic acid is a polyunsaturated omega-6 18 carbon long fatty acid, with two CC double bonds at the 9- and 12-positions. Thus, 15,16-dihode is considered to be an octadecanoid. Based on a literature review very few articles have been published on 15,16-DiHODE. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+/-)-15,16-dihydroxy-9Z,12Z-octadecadienoate | HMDB | | (+/-)-15,16-dihydroxy-9Z,12Z-octadecadienoic acid | HMDB | | (9Z,12Z)-15,16-Dihydroxyoctadeca-9,12-dienoate | HMDB | | (9Z,12Z)-15,16-Dihydroxyoctadeca-9,12-dienoic acid | HMDB | | a-15,16-DiHODE | HMDB | | alpha-15,16-DiHODE | HMDB | | Α-15,16-dihode | HMDB |
|
|---|
| Chemical Formula | C18H32O4 |
|---|
| Average Molecular Weight | 312.4443 |
|---|
| Monoisotopic Molecular Weight | 312.230059512 |
|---|
| IUPAC Name | (9Z,12Z)-15,16-dihydroxyoctadeca-9,12-dienoic acid |
|---|
| Traditional Name | (9Z,12Z)-15,16-dihydroxyoctadeca-9,12-dienoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CCC(O)C(O)C\C=C/C\C=C/CCCCCCCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C18H32O4/c1-2-16(19)17(20)14-12-10-8-6-4-3-5-7-9-11-13-15-18(21)22/h4,6,10,12,16-17,19-20H,2-3,5,7-9,11,13-15H2,1H3,(H,21,22)/b6-4-,12-10- |
|---|
| InChI Key | LKLLJYJTYPVCID-OHPMOLHNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as lineolic acids and derivatives. These are derivatives of lineolic acid. Lineolic acid is a polyunsaturated omega-6 18 carbon long fatty acid, with two CC double bonds at the 9- and 12-positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Lineolic acids and derivatives |
|---|
| Direct Parent | Lineolic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Octadecanoid
- Long-chain fatty acid
- Hydroxy fatty acid
- Unsaturated fatty acid
- Fatty acid
- Secondary alcohol
- 1,2-diol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0zfu-5490000000-cfa891ba34da849428a2 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-01ua-9543550000-420842853ca01bf42583 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0092000000-233fc66b26e966502a1d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-056s-2390000000-71f073f08ba3be3bbf45 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0bvu-9720000000-6c73bb25927de4dbc4bb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0059000000-4317ae075999e29d720b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0btc-3092000000-b909cb6ea5d58b6d2628 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9020000000-e8d5b3fae3b9c76d70fb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0059000000-ea195e424fb88e0333cc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9070000000-90478242bfc220c7cce2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9350000000-c48ce20a1afa54b282c8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002b-2291000000-0b2a1fd5c9e93ced98f5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-054k-5490000000-b3d576c57da4f9aa2f65 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0aou-9500000000-570599b2c53f8d95d94b | View in MoNA |
|---|
|
|---|