| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:01:27 UTC |
|---|
| Update Date | 2020-04-22 15:39:26 UTC |
|---|
| BMDB ID | BMDB0010209 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 15-HEPE |
|---|
| Description | 15-HEPE, also known as 15(R)-hepe, belongs to the class of organic compounds known as hydroxyeicosapentaenoic acids. These are eicosanoic acids with an attached hydroxyl group and five CC double bonds. Thus, 15-HEPE is considered to be an eicosanoid. Based on a literature review very few articles have been published on 15-HEPE. |
|---|
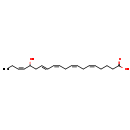
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (15R)-15-Hydroxyeicosa-5Z,8Z,11Z,13E,17Z-pentaenoate | HMDB | | (15R)-15-Hydroxyeicosa-5Z,8Z,11Z,13E,17Z-pentaenoic acid | HMDB | | (15S)-15-Hydroxyeicosa-5Z,8Z,11Z,13E,17Z-pentaenoate | HMDB | | (15S)-15-Hydroxyeicosa-5Z,8Z,11Z,13E,17Z-pentaenoic acid | HMDB | | 15(R)-HEPE | HMDB | | 15(S)-HEPE | HMDB | | 15-Hydroxy-5,8,11,13,17-eicosapentaenoate | HMDB | | 15-Hydroxy-5,8,11,13,17-eicosapentaenoic acid | HMDB | | 15-Hydroxyeicosapentaenoate | HMDB | | 15-Hydroxyeicosapentaenoic acid | HMDB |
|
|---|
| Chemical Formula | C20H30O3 |
|---|
| Average Molecular Weight | 318.4504 |
|---|
| Monoisotopic Molecular Weight | 318.219494826 |
|---|
| IUPAC Name | (5Z,8Z,11Z,13E,17Z)-16-hydroxyicosa-5,8,11,13,17-pentaenoic acid |
|---|
| Traditional Name | (5Z,8Z,11Z,13E,17Z)-16-hydroxyicosa-5,8,11,13,17-pentaenoic acid |
|---|
| CAS Registry Number | 97850-14-1 |
|---|
| SMILES | CC\C=C/C(O)C\C=C\C=C/C\C=C/C\C=C/CCCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C20H30O3/c1-2-3-16-19(21)17-14-12-10-8-6-4-5-7-9-11-13-15-18-20(22)23/h3-5,8-12,14,16,19,21H,2,6-7,13,15,17-18H2,1H3,(H,22,23)/b5-4-,10-8-,11-9-,14-12+,16-3- |
|---|
| InChI Key | UDXLGBLAJBYLSZ-XBCQTNLFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hydroxyeicosapentaenoic acids. These are eicosanoic acids with an attached hydroxyl group and five CC double bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Eicosanoids |
|---|
| Direct Parent | Hydroxyeicosapentaenoic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydroxyeicosapentaenoic acid
- Long-chain fatty acid
- Hydroxy fatty acid
- Fatty acid
- Unsaturated fatty acid
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic oxide
- Organic oxygen compound
- Alcohol
- Hydrocarbon derivative
- Carbonyl group
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0f6x-9182000000-ceafb87ef7f18bc914d6 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-05i1-9326300000-3419bfcd6eb3a12958b6 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0059000000-8dc15ca069de44682ca9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0piu-5393000000-745f15890cf2792b4b11 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05g3-9830000000-9e574ad248ec3d50dc13 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0059000000-34299018bad23c98aff4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05mk-2093000000-8a92b0e27bb95dea96cc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9040000000-b5e113a59ae2eb53aee4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0049000000-f5ec142a0faf404e6d4e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-1192000000-522c58a764f029147218 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9340000000-cc81888926ff0bf803fd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0459000000-c6826814cc998807d75c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ue9-3943000000-9e808383e7f699e97ccf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05nf-7910000000-5cafe34e26fdc4941799 | View in MoNA |
|---|
|
|---|