| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:04:39 UTC |
|---|
| Update Date | 2020-05-11 20:25:10 UTC |
|---|
| BMDB ID | BMDB0010339 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 3-alpha-androstanediol glucuronide |
|---|
| Description | 3-alpha-Androstanediol glucuronide belongs to the class of organic compounds known as steroid glucuronide conjugates. These are sterol lipids containing a glucuronide moiety linked to the steroid skeleton. Based on a literature review a significant number of articles have been published on 3-alpha-Androstanediol glucuronide. |
|---|
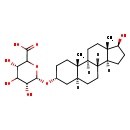
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-a-Androstanediol glucuronide | Generator | | 3-Α-androstanediol glucuronide | Generator | | Androstane-3alpha,17beta-diol 3-D-glucuronide | HMDB | | Androstane-3alpha,17beta-diol 3-delta-glucuronide | HMDB | | Androstane-3,17-diol glucuronide | MeSH, HMDB | | 3 alpha-Diol g | MeSH, HMDB | | 5-alpha-Androstane-3 alpha,17 beta-diol glucuronide | MeSH, HMDB | | Androstane-3,17-diol glucuronide, (3alpha,5beta,17beta)-isomer | MeSH, HMDB | | 3,17-Androstanediol glucuronide | MeSH, HMDB | | 3-Hydroxyandrostan-17-yl-glucopyranosiduronic acid | MeSH, HMDB | | Androstane-3,17-diol glucuronide, (3beta,5alpha,17beta)-isomer | MeSH, HMDB | | (3S,5R,6S)-3,4,5-Trihydroxy-6-{[(1S,2S,5R,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-5-yl]oxy}oxane-2-carboxylate | Generator | | 3-alpha-Androstanediol glucuronide | MeSH |
|
|---|
| Chemical Formula | C25H40O8 |
|---|
| Average Molecular Weight | 468.5803 |
|---|
| Monoisotopic Molecular Weight | 468.272318256 |
|---|
| IUPAC Name | (3S,5R,6S)-3,4,5-trihydroxy-6-{[(1S,2S,5R,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-5-yl]oxy}oxane-2-carboxylic acid |
|---|
| Traditional Name | 3-α-androstanediol glucuronide |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])C[C@@H](CC[C@]12C)O[C@H]1OC([C@@H](O)C(O)[C@H]1O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C25H40O8/c1-24-9-7-13(32-23-20(29)18(27)19(28)21(33-23)22(30)31)11-12(24)3-4-14-15-5-6-17(26)25(15,2)10-8-16(14)24/h12-21,23,26-29H,3-11H2,1-2H3,(H,30,31)/t12-,13+,14-,15-,16-,17-,18?,19-,20+,21?,23-,24-,25-/m0/s1 |
|---|
| InChI Key | GYNWSIBKBBWJJW-YMRFBRODSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as steroid glucuronide conjugates. These are sterol lipids containing a glucuronide moiety linked to the steroid skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Steroidal glycosides |
|---|
| Direct Parent | Steroid glucuronide conjugates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Steroid-glucuronide-skeleton
- Androstane-skeleton
- 17-hydroxysteroid
- Hydroxysteroid
- O-glucuronide
- 1-o-glucuronide
- Glucuronic acid or derivatives
- Glycosyl compound
- O-glycosyl compound
- Beta-hydroxy acid
- Hydroxy acid
- Pyran
- Oxane
- Monosaccharide
- Cyclic alcohol
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Polyol
- Acetal
- Organoheterocyclic compound
- Oxacycle
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Organic oxygen compound
- Alcohol
- Organooxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0ug0-4132900000-0bc546d56d6293b9fcf3 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-00xr-3405029000-7fb31a295ed378f2edcf | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fvl-0090600000-c23895c57f9e6efc3831 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004l-0190000000-0ff1cc081b770a48292f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ta-0590000000-9cafc36a711636e7d84c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01bc-1160900000-9d71d7317d152fba5d86 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00dl-1290300000-7f46ecf0d6e040be76ec | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-3190000000-9ffaff29eb395b5bb61d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0010900000-f00f8e0d5befb993dec0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6r-1892300000-87689c41c4589227c1d2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4j-1914100000-f49edf2061e78a78bf4e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000900000-c41d4bc10932b62a1eec | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-2221900000-85e2ee343d862c76be90 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0abi-9243100000-826ed7e7dc561ef6cb76 | View in MoNA |
|---|
|
|---|