| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:04:48 UTC |
|---|
| Update Date | 2020-05-11 20:25:15 UTC |
|---|
| BMDB ID | BMDB0010346 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Triiodothyronine glucuronide |
|---|
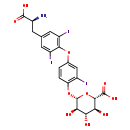
| Description | Triiodothyronine glucuronide, also known as T3G, belongs to the class of organic compounds known as phenylalanine and derivatives. Phenylalanine and derivatives are compounds containing phenylalanine or a derivative thereof resulting from reaction of phenylalanine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. Triiodothyronine glucuronide is a very strong basic compound (based on its pKa). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (S)-4-(4-(2-Amino-2-carboxyethyl)-2,6-diiodophenoxy)-2-iodophenyl-beta-D-glucopyranosiduronic acid | HMDB | | (S)-4-(4-(2-Amino-2-carboxyethyl)-2,6-diiodophenoxy)-2-iodophenyl-beta-delta-glucopyranosiduronic acid | HMDB | | Triiodothyronine glucuronoside | HMDB | | T3G | HMDB | | (2S,3S,4S,5R,6S)-6-(4-{4-[(2S)-2-amino-2-carboxyethyl]-2,6-diiodophenoxy}-2-iodophenoxy)-3,4,5-trihydroxyoxane-2-carboxylate | HMDB | | 3,3',5-Triiodo-L-thyronine 4'-O-beta-D-glucuronide | HMDB | | 3,3',5-Triiodo-L-thyronine 4'-O-β-D-glucuronide | HMDB | | 3,3',5-Triiodo-L-thyronine O-beta-D-glucuronide | HMDB | | 3,3',5-Triiodothyronine O-beta-D-glucuronide | HMDB | | 3,3',5-Triiodothyronine glucuronide | HMDB | | 3,3,5-Triiodo-L-thyronine-beta-D-glucuronoside | HMDB | | 3,3,5-Triiodo-L-thyronine-β-D-glucuronoside | HMDB | | 3,3’,5-triiodo-L-thyronine 4’-O-β-D-glucuronide | HMDB | | 3,3’,5-triiodo-L-thyronine O-β-D-glucuronide | HMDB | | 3,3’,5-triiodothyronine O-β-D-glucuronide | HMDB | | 3,3’,5-triiodothyronine glucuronide | HMDB | | 3,5,3'-Triiodothyronine glucuronide | HMDB | | 3,5,3’-triiodothyronine glucuronide | HMDB | | Triiodothyronine O-beta-D-glucuronide | HMDB | | Triiodothyronine O-β-D-glucuronide | HMDB | | Triiodothyronine glucuronide | MeSH |
|
|---|
| Chemical Formula | C21H20I3NO10 |
|---|
| Average Molecular Weight | 827.0976 |
|---|
| Monoisotopic Molecular Weight | 826.822126125 |
|---|
| IUPAC Name | (2S,3S,4S,5R,6S)-6-(4-{4-[(2S)-2-amino-2-carboxyethyl]-2,6-diiodophenoxy}-2-iodophenoxy)-3,4,5-trihydroxyoxane-2-carboxylic acid |
|---|
| Traditional Name | triiodothyronine glucuronide |
|---|
| CAS Registry Number | 29919-72-0 |
|---|
| SMILES | N[C@@H](CC1=CC(I)=C(OC2=CC(I)=C(O[C@@H]3O[C@@H]([C@@H](O)[C@H](O)[C@H]3O)C(O)=O)C=C2)C(I)=C1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C21H20I3NO10/c22-9-6-8(33-17-10(23)3-7(4-11(17)24)5-12(25)19(29)30)1-2-13(9)34-21-16(28)14(26)15(27)18(35-21)20(31)32/h1-4,6,12,14-16,18,21,26-28H,5,25H2,(H,29,30)(H,31,32)/t12-,14-,15-,16+,18-,21+/m0/s1 |
|---|
| InChI Key | YYFGGGCINNGOLE-ZDXOGFQLSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylalanine and derivatives. Phenylalanine and derivatives are compounds containing phenylalanine or a derivative thereof resulting from reaction of phenylalanine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Phenylalanine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylalanine or derivatives
- Phenolic glycoside
- O-glucuronide
- 1-o-glucuronide
- Diphenylether
- Glucuronic acid or derivatives
- Hexose monosaccharide
- 3-phenylpropanoic-acid
- O-glycosyl compound
- Glycosyl compound
- Diaryl ether
- Amphetamine or derivatives
- L-alpha-amino acid
- Alpha-amino acid
- Phenoxy compound
- Phenol ether
- Halobenzene
- Aralkylamine
- Iodobenzene
- Beta-hydroxy acid
- Aryl halide
- Aryl iodide
- Monocyclic benzene moiety
- Dicarboxylic acid or derivatives
- Hydroxy acid
- Benzenoid
- Monosaccharide
- Pyran
- Oxane
- Secondary alcohol
- Amino acid
- Acetal
- Polyol
- Carboxylic acid
- Organoheterocyclic compound
- Oxacycle
- Ether
- Primary aliphatic amine
- Hydrocarbon derivative
- Amine
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Organopnictogen compound
- Alcohol
- Primary amine
- Organohalogen compound
- Organoiodide
- Organonitrogen compound
- Organooxygen compound
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0pc0-0000009660-4b8db75411fb4ad3840b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0zgi-0000009100-ae2bd193ecb19a0b6425 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0uxr-0133159000-b987c4874aea3d80ebd5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-005a-2200006490-37ebd7b467add975a4c4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000t-2200009210-1fc55e521a5e081aca43 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-007k-5100119000-cea3decba2c3578e5a88 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a7i-0100000590-8de78883eece517fca16 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0lz9-0000004920-2e31e12e0f11a4836060 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fc0-3900017710-32e7a7f1dae3ad9b7e3d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000000090-df43da69d824b61621e1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004j-5700009220-eced4acd734d2fb4572e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-1900000000-5f4728a2997b91c4a444 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|