| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:12:30 UTC |
|---|
| Update Date | 2020-05-21 16:27:14 UTC |
|---|
| BMDB ID | BMDB0010720 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | But-2-enoic acid |
|---|
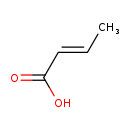
| Description | But-2-enoic acid, also known as but-2-enoic acid or (2E)-But-2-enoic acid, belongs to the class of organic compounds known as straight chain fatty acids. These are fatty acids with a straight aliphatic chain. But-2-enoic acid exists as a solid, very hydrophobic, practically insoluble (in water), and relatively neutral molecule. But-2-enoic acid exists in all living species, ranging from bacteria to humans. But-2-enoic acid participates in a number of enzymatic reactions, within cattle. In particular, But-2-enoic acid can be biosynthesized from 3-hydroxybutyric acid; which is catalyzed by the enzyme fatty acid synthase. dyhydrase domain. In addition, But-2-enoic acid can be converted into butyric acid through its interaction with the enzyme fatty acid synthase. enoyl reductase domain. In cattle, but-2-enoic acid is involved in the metabolic pathway called fatty acid biosynthesis pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2E)-2-Butenoic acid | ChEBI | | (e)-2-Butenoic acid | ChEBI | | (e)-But-2-enoic acid | ChEBI | | (e)-Crotonic acid | ChEBI | | 2-Butenoic acid | ChEBI | | 3-Methylacrylic acid | ChEBI | | alpha-Butenoic acid | ChEBI | | alpha-Crotonic acid | ChEBI | | BEO | ChEBI | | beta-Methacrylic acid | ChEBI | | beta-Methylacrylic acid | ChEBI | | trans-2-Butenoic acid | ChEBI | | trans-Crotonic acid | ChEBI | | Crotonic acid | Kegg | | (2E)-2-Butenoate | Generator | | (e)-2-Butenoate | Generator | | (e)-But-2-enoate | Generator | | (e)-Crotonate | Generator | | 2-Butenoate | Generator | | 3-Methylacrylate | Generator | | a-Butenoate | Generator | | a-Butenoic acid | Generator | | alpha-Butenoate | Generator | | Α-butenoate | Generator | | Α-butenoic acid | Generator | | a-Crotonate | Generator | | a-Crotonic acid | Generator | | alpha-Crotonate | Generator | | Α-crotonate | Generator | | Α-crotonic acid | Generator | | b-Methacrylate | Generator | | b-Methacrylic acid | Generator | | beta-Methacrylate | Generator | | Β-methacrylate | Generator | | Β-methacrylic acid | Generator | | b-Methylacrylate | Generator | | b-Methylacrylic acid | Generator | | beta-Methylacrylate | Generator | | Β-methylacrylate | Generator | | Β-methylacrylic acid | Generator | | trans-2-Butenoate | Generator | | trans-Crotonate | Generator | | Crotonate | Generator | | But-2-enoate | Generator | | (2E)-But-2-enoate | HMDB | | (2E)-But-2-enoic acid | HMDB | | 3-Methyl-acrylic acid | HMDB | | Butenoate | HMDB | | Butenoic acid | HMDB | | Kyselina krotonova | HMDB | | Solid crotonic acid | HMDB |
|
|---|
| Chemical Formula | C4H6O2 |
|---|
| Average Molecular Weight | 86.0892 |
|---|
| Monoisotopic Molecular Weight | 86.036779436 |
|---|
| IUPAC Name | (2E)-but-2-enoic acid |
|---|
| Traditional Name | butenoic acid |
|---|
| CAS Registry Number | 3724-65-0 |
|---|
| SMILES | C\C=C\C(O)=O |
|---|
| InChI Identifier | InChI=1S/C4H6O2/c1-2-3-4(5)6/h2-3H,1H3,(H,5,6)/b3-2+ |
|---|
| InChI Key | LDHQCZJRKDOVOX-NSCUHMNNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as straight chain fatty acids. These are fatty acids with a straight aliphatic chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
| Direct Parent | Straight chain fatty acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Unsaturated fatty acid
- Straight chain fatty acid
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Adiposome
- Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 72 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | 0.72 | HANSCH,C ET AL. (1995) |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-000l-9000000000-c066ee0b8aa28cc564f5 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-000l-9000000000-c066ee0b8aa28cc564f5 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000f-9000000000-dcef1149fbd6447033ad | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0103-9100000000-bb87072a7225402c6d0e | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-000i-9000000000-139e86f163451c657b84 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-000i-9000000000-b1e1c9a0bcfd328b1cd9 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0006-9000000000-e0d8bcef7823a57ace6b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0006-9000000000-f496e2b96544aa9df21d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-000f-9000000000-5905542348788794febc | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-b58156e5b9ec6e1ed71e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9000000000-14c905772071113e3bef | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-9000000000-1dc3bc86132e73942861 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00ko-9000000000-d17c17c2c66a97ee9c27 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-4cd5b916c7ee6039effc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-9000000000-dde621f263ff3ac72841 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000l-9000000000-dce352f985fb5e143263 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kf-9000000000-acd6176b8c6ebe251635 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-9000000000-ec20127c74818b1f634d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00kr-9000000000-acc7f4424bb0833d9f87 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014l-9000000000-f6cecaee0aa536b15160 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kf-9000000000-1462d04bf056a8a82ccd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-9000000000-210cae383690d066d0e0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-1aa1299c9e5efe8dc421 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 25.16 MHz, CDCl3, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|