| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:12:38 UTC |
|---|
| Update Date | 2020-05-21 16:27:15 UTC |
|---|
| BMDB ID | BMDB0010728 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | (R)-3-Hydroxydodecanoic acid |
|---|
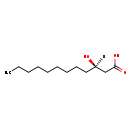
| Description | (R)-3-Hydroxydodecanoic acid, also known as (3R)-3-hydroxylauric acid or (R)-beta-OH lauric acid, belongs to the class of organic compounds known as medium-chain hydroxy acids and derivatives. These are hydroxy acids with a 6 to 12 carbon atoms long side chain (R)-3-Hydroxydodecanoic acid is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral (R)-3-Hydroxydodecanoic acid exists in all eukaryotes, ranging from yeast to humans (R)-3-Hydroxydodecanoic acid participates in a number of enzymatic reactions, within cattle. In particular, (R)-3-Hydroxydodecanoic acid can be biosynthesized from 3-oxododecanoic acid through its interaction with the enzyme fatty acid synthase. Beta ketoacyl synthase domain. In addition, (R)-3-Hydroxydodecanoic acid can be converted into trans-dodec-2-enoic acid; which is catalyzed by the enzyme fatty acid synthase. dyhydrase domain. In cattle, (R)-3-hydroxydodecanoic acid is involved in the metabolic pathway called fatty acid biosynthesis pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3R)-3-Hydroxydodecanoic acid | ChEBI | | (3R)-3-Hydroxylauric acid | ChEBI | | (R)-3-OH Dodecanoic acid | ChEBI | | (R)-3-OH Lauric acid | ChEBI | | (R)-beta-Hydroxydodecanoic acid | ChEBI | | (R)-beta-Hydroxylauric acid | ChEBI | | (R)-beta-OH Dodecanoic acid | ChEBI | | (R)-beta-OH Lauric acid | ChEBI | | D-(-)-3-Hydroxydodecanoic acid | ChEBI | | D-3-Hydroxydodecanoic acid | ChEBI | | (3R)-3-Hydroxydodecanoate | Generator | | (3R)-3-Hydroxylaate | Generator | | (3R)-3-Hydroxylaic acid | Generator | | (R)-3-OH Dodecanoate | Generator | | (R)-3-OH Laate | Generator | | (R)-3-OH Laic acid | Generator | | (R)-b-Hydroxydodecanoate | Generator | | (R)-b-Hydroxydodecanoic acid | Generator | | (R)-beta-Hydroxydodecanoate | Generator | | (R)-Β-hydroxydodecanoate | Generator | | (R)-Β-hydroxydodecanoic acid | Generator | | (R)-b-Hydroxylaate | Generator | | (R)-b-Hydroxylaic acid | Generator | | (R)-beta-Hydroxylaate | Generator | | (R)-beta-Hydroxylaic acid | Generator | | (R)-Β-hydroxylaate | Generator | | (R)-Β-hydroxylaic acid | Generator | | (R)-b-OH Dodecanoate | Generator | | (R)-b-OH Dodecanoic acid | Generator | | (R)-beta-OH Dodecanoate | Generator | | (R)-Β-OH dodecanoate | Generator | | (R)-Β-OH dodecanoic acid | Generator | | (R)-b-OH Laate | Generator | | (R)-b-OH Laic acid | Generator | | (R)-beta-OH Laate | Generator | | (R)-beta-OH Laic acid | Generator | | (R)-Β-OH laate | Generator | | (R)-Β-OH laic acid | Generator | | D-(-)-3-Hydroxydodecanoate | Generator | | D-3-Hydroxydodecanoate | Generator | | (R)-3-Hydroxydodecanoate | Generator |
|
|---|
| Chemical Formula | C12H24O3 |
|---|
| Average Molecular Weight | 216.3172 |
|---|
| Monoisotopic Molecular Weight | 216.172544634 |
|---|
| IUPAC Name | (3R)-3-hydroxydodecanoic acid |
|---|
| Traditional Name | (R)-3-hydroxydodecanoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@@](O)(CCCCCCCCC)CC(O)=O |
|---|
| InChI Identifier | InChI=1S/C12H24O3/c1-2-3-4-5-6-7-8-9-11(13)10-12(14)15/h11,13H,2-10H2,1H3,(H,14,15)/t11-/m1/s1 |
|---|
| InChI Key | MUCMKTPAZLSKTL-LLVKDONJSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as medium-chain hydroxy acids and derivatives. These are hydroxy acids with a 6 to 12 carbon atoms long side chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Hydroxy acids and derivatives |
|---|
| Sub Class | Medium-chain hydroxy acids and derivatives |
|---|
| Direct Parent | Medium-chain hydroxy acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Medium-chain hydroxy acid
- Medium-chain fatty acid
- Beta-hydroxy acid
- Hydroxy fatty acid
- Fatty acyl
- Fatty acid
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic oxide
- Alcohol
- Carbonyl group
- Hydrocarbon derivative
- Organic oxygen compound
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000f-9500000000-ce0e4abc46c623ad5053 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00bc-9042000000-0d213d881baf293e004c | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kb-0930000000-57fa523571a510c84940 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f7k-3900000000-db0ac4f7e5c200e8ffe9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9200000000-af4ea991e8885d84f4d2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-1790000000-438c129b69517f0cd6f1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0q29-3920000000-f4f68c93f91c4519ee51 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9600000000-05e66928d4652560bee3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-1190000000-5af11549237f298c2ea2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9210000000-0ad9609790293200ce6e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9200000000-abdab144438d186cdcc0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014j-8950000000-eef6079ee3aba96447b9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9200000000-2cc48879a8d7f0de8899 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9000000000-b899673a8a3334e8f36a | View in MoNA |
|---|
|
|---|