| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:12:41 UTC |
|---|
| Update Date | 2020-05-21 16:27:15 UTC |
|---|
| BMDB ID | BMDB0010731 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | (R)-3-Hydroxy-tetradecanoic acid |
|---|
| Description | (R)-3-Hydroxy-tetradecanoic acid, also known as (3R)-hydroxymyristic acid or (r)-3-hydroxy-tetradecanoic acid, belongs to the class of organic compounds known as long-chain fatty acids. These are fatty acids with an aliphatic tail that contains between 13 and 21 carbon atoms (R)-3-Hydroxy-tetradecanoic acid is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral (R)-3-Hydroxy-tetradecanoic acid exists in all eukaryotes, ranging from yeast to humans (R)-3-Hydroxy-tetradecanoic acid participates in a number of enzymatic reactions, within cattle. In particular, (R)-3-Hydroxy-tetradecanoic acid can be biosynthesized from 3-oxotetradecanoic acid through the action of the enzyme fatty acid synthase. Beta ketoacyl synthase domain. In addition, (R)-3-Hydroxy-tetradecanoic acid can be converted into trans-tetra-dec-2-enoic acid; which is mediated by the enzyme fatty acid synthase. dyhydrase domain. In cattle, (R)-3-hydroxy-tetradecanoic acid is involved in the metabolic pathway called fatty acid biosynthesis pathway. |
|---|
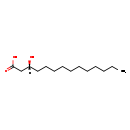
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (R)-3-Hydroxy-tetradecanoate | Generator | | (-)-3-Hydroxymyristic acid | HMDB | | (3R)-Hydroxymyristic acid | HMDB | | (R)-(-)-3-Hydroxytetradecanoic acid | HMDB | | (R)-3-Hydroxymyristic acid | HMDB | | beta-Hydroxymyristic acid | HMDB | | beta-Hydroxytetradecanoic acid | HMDB | | D-(-)-beta-Hydroxymyristic acid | HMDB | | (-)-3-Hydroxymyristate | HMDB | | (3R)-Hydroxymyristate | HMDB | | (R)-(-)-3-Hydroxytetradecanoate | HMDB | | (R)-3-Hydroxymyristate | HMDB | | b-Hydroxymyristate | HMDB | | b-Hydroxymyristic acid | HMDB | | beta-Hydroxymyristate | HMDB | | Β-hydroxymyristate | HMDB | | Β-hydroxymyristic acid | HMDB | | b-Hydroxytetradecanoate | HMDB | | b-Hydroxytetradecanoic acid | HMDB | | beta-Hydroxytetradecanoate | HMDB | | Β-hydroxytetradecanoate | HMDB | | Β-hydroxytetradecanoic acid | HMDB | | D-(-)-b-Hydroxymyristate | HMDB | | D-(-)-b-Hydroxymyristic acid | HMDB | | D-(-)-beta-Hydroxymyristate | HMDB | | D-(-)-Β-hydroxymyristate | HMDB | | D-(-)-Β-hydroxymyristic acid | HMDB | | (R)-3-Hydroxytetradecanoic acid | HMDB | | (R)-3-Hydroxytetradecanoate | HMDB |
|
|---|
| Chemical Formula | C14H28O3 |
|---|
| Average Molecular Weight | 244.3703 |
|---|
| Monoisotopic Molecular Weight | 244.203844762 |
|---|
| IUPAC Name | (3R)-3-hydroxytetradecanoic acid |
|---|
| Traditional Name | 3-hydroxy-tetradecanoic acid |
|---|
| CAS Registry Number | 28715-21-1 |
|---|
| SMILES | [H][C@@](O)(CCCCCCCCCCC)CC(O)=O |
|---|
| InChI Identifier | InChI=1S/C14H28O3/c1-2-3-4-5-6-7-8-9-10-11-13(15)12-14(16)17/h13,15H,2-12H2,1H3,(H,16,17)/t13-/m1/s1 |
|---|
| InChI Key | ATRNZOYKSNPPBF-CYBMUJFWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as long-chain fatty acids. These are fatty acids with an aliphatic tail that contains between 13 and 21 carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
| Direct Parent | Long-chain fatty acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Long-chain fatty acid
- Hydroxy fatty acid
- Beta-hydroxy acid
- Hydroxy acid
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Adiposome
- Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000f-9500000000-d3837d6527b09790d09e | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-05fr-9142000000-2b802003b701914b13b7 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004j-0290000000-3cbad25621cd84dd55dc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-057j-4960000000-28121e72e39700d4f373 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9300000000-77f13667a638fc0db127 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-1490000000-041e325e3e96f0f26837 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a5d-4950000000-ceff7e67442b0ca855ab | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9600000000-2a651411e77e87c314c5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-1090000000-576941859c2295d4cb14 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9110000000-be27ca1efc0330f18073 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9110000000-b5cfa3eff2f2e135cf70 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002b-4390000000-0cbb960da18db3e0e662 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0pb9-9310000000-0ff6d44ab8d422fb3b9b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-29ad8b4130f67f29eb80 | View in MoNA |
|---|
|
|---|