| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:22:17 UTC |
|---|
| Update Date | 2020-04-22 15:45:46 UTC |
|---|
| BMDB ID | BMDB0011651 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
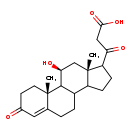
| Common Name | 11beta,20-Dihydroxy-3-oxopregn-4-en-21-oic acid |
|---|
| Description | 11beta,20-Dihydroxy-3-oxopregn-4-en-21-oic acid belongs to the class of organic compounds known as 20-oxosteroids. These are steroid derivatives carrying a C=O group at the 20-position of the steroid skeleton. Based on a literature review a small amount of articles have been published on 11beta,20-Dihydroxy-3-oxopregn-4-en-21-oic acid. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 11b,20-Dihydroxy-3-oxopregn-4-en-21-Oate | Generator | | 11b,20-Dihydroxy-3-oxopregn-4-en-21-Oic acid | Generator | | 11beta,20-Dihydroxy-3-oxopregn-4-en-21-Oate | Generator | | 11Β,20-dihydroxy-3-oxopregn-4-en-21-Oate | Generator | | 11Β,20-dihydroxy-3-oxopregn-4-en-21-Oic acid | Generator | | DHOPA | HMDB | | 3-[(2R,15S,17S)-17-Hydroxy-2,15-dimethyl-5-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-6-en-14-yl]-3-oxopropanoate | Generator |
|
|---|
| Chemical Formula | C22H30O5 |
|---|
| Average Molecular Weight | 374.4706 |
|---|
| Monoisotopic Molecular Weight | 374.20932407 |
|---|
| IUPAC Name | 3-[(2R,15S,17S)-17-hydroxy-2,15-dimethyl-5-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-6-en-14-yl]-3-oxopropanoic acid |
|---|
| Traditional Name | 3-[(2R,15S,17S)-17-hydroxy-2,15-dimethyl-5-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-6-en-14-yl]-3-oxopropanoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | C[C@]12C[C@H](O)C3C(CCC4=CC(=O)CC[C@]34C)C1CCC2C(=O)CC(O)=O |
|---|
| InChI Identifier | InChI=1S/C22H30O5/c1-21-8-7-13(23)9-12(21)3-4-14-15-5-6-16(17(24)10-19(26)27)22(15,2)11-18(25)20(14)21/h9,14-16,18,20,25H,3-8,10-11H2,1-2H3,(H,26,27)/t14?,15?,16?,18-,20?,21-,22-/m0/s1 |
|---|
| InChI Key | VUDYWBBYQWVFAG-GKLBWGGBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 20-oxosteroids. These are steroid derivatives carrying a C=O group at the 20-position of the steroid skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Oxosteroids |
|---|
| Direct Parent | 20-oxosteroids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 20-oxosteroid
- Androgen-skeleton
- Steroid acid
- Androstane-skeleton
- 3-oxo-delta-4-steroid
- 3-oxosteroid
- 11-hydroxysteroid
- 11-beta-hydroxysteroid
- Hydroxysteroid
- Delta-4-steroid
- Cyclohexenone
- Beta-keto acid
- 1,3-dicarbonyl compound
- Beta-hydroxy ketone
- Keto acid
- Cyclic alcohol
- Secondary alcohol
- Cyclic ketone
- Ketone
- Carboxylic acid
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Organooxygen compound
- Carbonyl group
- Organic oxide
- Organic oxygen compound
- Hydrocarbon derivative
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0aor-3978000000-fc2bf2ea9193dcfa637f | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0udi-3224690000-d03adcaf1bea60c6623e | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-0009000000-a897ef38166e76a94688 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-08i9-1119000000-3341805bfe9a08acef72 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0aor-6594000000-60b3133186ed94c1368a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-05i0-0009000000-fcc060ebc9b15e90993d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-08i0-1009000000-1fd24a5a0643042de1bd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0bvl-8039000000-5743c463c4e82394d2d6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0009000000-042597955647d9bccff2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0c0c-5049000000-ff5e69651fe9a0858f8a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006x-7296000000-a3b09436fd9015e16538 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05p9-0059000000-2f3c5f271f31708ff580 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014r-0192000000-1741031eb88f7b1da9d5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0300-2690000000-3a7431ed1feec7b18880 | View in MoNA |
|---|
|
|---|