| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:22:24 UTC |
|---|
| Update Date | 2020-04-22 15:45:48 UTC |
|---|
| BMDB ID | BMDB0011657 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
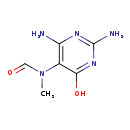
| Common Name | 2,6-Diamino-4-hydroxy-5-N-methylformamidopyrimidine |
|---|
| Description | 2,6-Diamino-4-hydroxy-5-N-methylformamidopyrimidine belongs to the class of organic compounds known as hydroxypyrimidines. These are organic compounds containing a hydroxyl group attached to a pyrimidine ring. Pyrimidine is a 6-membered ring consisting of four carbon atoms and two nitrogen centers at the 1- and 3- ring positions. Based on a literature review very few articles have been published on 2,6-Diamino-4-hydroxy-5-N-methylformamidopyrimidine. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,6-Diamino-4-hydroxy-5-(N-methyl)formamidopyrimidine | ChEBI | | 2,6-diamino-4-Hydroxy- 5- formamidopyrimidine | HMDB | | Me-fapygua | HMDB | | N-(2,4-diamino-1,6-dihydro-6-oxo-5-Pyrimidinyl)-N-methyl-formamide | HMDB | | N5-Methyl-N5-formyl-2,5,6-triamino-4-hydroxypyrimidine | HMDB | | MFTAHP | MeSH, HMDB | | N(5)-Methyl-N(5)-formyl-2,5,6-triamino-4-hydroxypyrimidine | MeSH, HMDB | | 2,6-Diamino-4-hydroxy-5-N-methylformamidopyrimidine | MeSH |
|
|---|
| Chemical Formula | C6H9N5O2 |
|---|
| Average Molecular Weight | 183.168 |
|---|
| Monoisotopic Molecular Weight | 183.075624557 |
|---|
| IUPAC Name | N-(2,4-diamino-6-hydroxypyrimidin-5-yl)-N-methylformamide |
|---|
| Traditional Name | N-(2,4-diamino-6-hydroxypyrimidin-5-yl)-N-methylformamide |
|---|
| CAS Registry Number | 77440-13-2 |
|---|
| SMILES | CN(C=O)C1=C(N)N=C(N)N=C1O |
|---|
| InChI Identifier | InChI=1S/C6H9N5O2/c1-11(2-12)3-4(7)9-6(8)10-5(3)13/h2H,1H3,(H5,7,8,9,10,13) |
|---|
| InChI Key | CGWDNAFNQOBSCK-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hydroxypyrimidines. These are organic compounds containing a hydroxyl group attached to a pyrimidine ring. Pyrimidine is a 6-membered ring consisting of four carbon atoms and two nitrogen centers at the 1- and 3- ring positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazines |
|---|
| Sub Class | Pyrimidines and pyrimidine derivatives |
|---|
| Direct Parent | Hydroxypyrimidines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aminopyrimidine
- Hydroxypyrimidine
- Hydropyrimidine
- Tertiary carboxylic acid amide
- Heteroaromatic compound
- Amino acid or derivatives
- Carboxamide group
- Azacycle
- Carboxylic acid derivative
- Organopnictogen compound
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Amine
- Carbonyl group
- Organic nitrogen compound
- Organic oxide
- Hydrocarbon derivative
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0kai-2900000000-a67d7f80d254667fa8ae | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0itd-1390000000-7af95ca919277dda70d8 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-053r-0900000000-161782a982bd9e0f746b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a59-0900000000-8aabbbca1867aa5ee470 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-9200000000-79543490b30fc623afa9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0900000000-1a59e57626ce37c5638e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0w29-2900000000-e8832d2c064b26923301 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9300000000-78862d713f4e4a809290 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-053r-0900000000-7cddc5f02d68b5f58fe5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0540-0900000000-1299b98119aa35f053c7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-007d-9200000000-3b5b503fd38c06191869 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0900000000-c5ca8ed84c0281e2cfb3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0gw0-3900000000-4ce1f6206fd7400e51ec | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9200000000-afeccf534860ddaa88be | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|