| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:23:25 UTC |
|---|
| Update Date | 2020-04-22 15:46:07 UTC |
|---|
| BMDB ID | BMDB0011714 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Vanilpyruvic acid |
|---|
| Description | Vanilpyruvic acid, also known as vanilpyruvate or HMPPA, belongs to the class of organic compounds known as phenylpyruvic acid derivatives. Phenylpyruvic acid derivatives are compounds containing a phenylpyruvic acid moiety, which consists of a phenyl group substituted at the second position by an pyruvic acid. Vanilpyruvic acid, with regard to humans, has been linked to the inborn metabolic disorder aromatic l-amino acid decarboxylase deficiency. Based on a literature review very few articles have been published on Vanilpyruvic acid. |
|---|
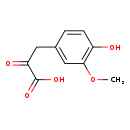
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Vanilpyruvate | Generator | | 3-Methoxy-4-hydroxyphenylpyruvic acid | MeSH | | 4-Hydroxy-3-methoxyphenylpyruvic acid | MeSH | | HMPPA | MeSH | | Vanilpyruvic acid, (14)C-labeled | MeSH | | 3-(4-Hydroxy-3-methoxyphenyl)-2-oxopropanoate | Generator, HMDB | | Vanilpyruvic acid | MeSH |
|
|---|
| Chemical Formula | C10H10O5 |
|---|
| Average Molecular Weight | 210.1834 |
|---|
| Monoisotopic Molecular Weight | 210.05282343 |
|---|
| IUPAC Name | 3-(4-hydroxy-3-methoxyphenyl)-2-oxopropanoic acid |
|---|
| Traditional Name | 3-(4-hydroxy-3-methoxyphenyl)-2-oxopropanoic acid |
|---|
| CAS Registry Number | 1081-71-6 |
|---|
| SMILES | COC1=C(O)C=CC(CC(=O)C(O)=O)=C1 |
|---|
| InChI Identifier | InChI=1S/C10H10O5/c1-15-9-5-6(2-3-7(9)11)4-8(12)10(13)14/h2-3,5,11H,4H2,1H3,(H,13,14) |
|---|
| InChI Key | YGQHQTMRZPHIBB-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylpyruvic acid derivatives. Phenylpyruvic acid derivatives are compounds containing a phenylpyruvic acid moiety, which consists of a phenyl group substituted at the second position by an pyruvic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Phenylpyruvic acid derivatives |
|---|
| Direct Parent | Phenylpyruvic acid derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylpyruvate
- 3-phenylpropanoic-acid
- Methoxyphenol
- Phenoxy compound
- Methoxybenzene
- Phenol ether
- Anisole
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Phenol
- Alpha-keto acid
- Keto acid
- Alpha-hydroxy ketone
- Ketone
- Carboxylic acid derivative
- Carboxylic acid
- Ether
- Monocarboxylic acid or derivatives
- Carbonyl group
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-3900000000-dafbcec2d38954fef85e | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00ri-9432000000-3d55712bbfe9bdc7e323 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-029f-0930000000-0b172389e3c947156f73 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-029l-0900000000-ad02a787e85a9f49fb5e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052r-2900000000-940a4f4bf06342206ef6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-1590000000-e50dd99429a7ccc4dc9c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-07bf-1910000000-34be49b6f1e377c3b80a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01ot-2900000000-38792c690b55d020d815 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0ab9-5490000000-87ff334d9c765af556b7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-1900000000-9a789246b1b70fb792b7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006y-5900000000-41ded4bdb04a6a46395b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01p9-0940000000-063224a36815c236d0d3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052r-0900000000-b1b3201ed43aec49a25a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4r-4900000000-98f11828d3fafa8b3c11 | View in MoNA |
|---|
|
|---|