| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:23:43 UTC |
|---|
| Update Date | 2020-04-22 15:46:13 UTC |
|---|
| BMDB ID | BMDB0011732 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 2-Keto-L-gluconate |
|---|
| Description | 2-Keto-L-gluconate, also known as 2-dehydro-L-idonate or 2-ketoidonate, belongs to the class of organic compounds known as sugar acids and derivatives. Sugar acids and derivatives are compounds containing a saccharide unit which bears a carboxylic acid group. Based on a literature review very few articles have been published on 2-Keto-L-gluconate. |
|---|
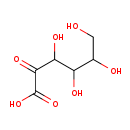
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Keto-L-gluconic acid | Generator | | 2-Dehydro-D-gluconate | HMDB | | 2-Dehydro-L-idonate | HMDB | | 2-Keto-D-gluconate | HMDB | | 2-Keto-L-gulonate | HMDB | | 2-Ketoidonate | HMDB | | 2-Oxogluconic acid | HMDB | | L-Ketoidonate | HMDB | | L-Ketoidonic acid | HMDB | | L-Sorbosonate | HMDB | | L-Sorbosonic acid | HMDB | | Provitamin C | HMDB | | Provitamin C, monosodium salt | HMDB | | 2-Keto-L-gulonic acid | HMDB | | Provitamin C, (L-xylo)-isomer | HMDB | | 2-Dehydro-L-gluconate | Generator |
|
|---|
| Chemical Formula | C6H10O7 |
|---|
| Average Molecular Weight | 194.1394 |
|---|
| Monoisotopic Molecular Weight | 194.042652674 |
|---|
| IUPAC Name | 3,4,5,6-tetrahydroxy-2-oxohexanoic acid |
|---|
| Traditional Name | 2-keto-D-gluconic acid |
|---|
| CAS Registry Number | 91548-32-2 |
|---|
| SMILES | OCC(O)C(O)C(O)C(=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C6H10O7/c7-1-2(8)3(9)4(10)5(11)6(12)13/h2-4,7-10H,1H2,(H,12,13) |
|---|
| InChI Key | VBUYCZFBVCCYFD-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sugar acids and derivatives. Sugar acids and derivatives are compounds containing a saccharide unit which bears a carboxylic acid group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Sugar acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hexose monosaccharide
- Medium-chain keto acid
- Beta-hydroxy acid
- Sugar acid
- Acyloin
- Alpha-keto acid
- Beta-hydroxy ketone
- Hydroxy acid
- Keto acid
- Monosaccharide
- Alpha-hydroxy ketone
- Ketone
- Secondary alcohol
- Carboxylic acid
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Polyol
- Alcohol
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Primary alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-08gi-9200000000-d157f971c8f1543d621c | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (5 TMS) - 70eV, Positive | splash10-05p9-5222950000-e80e85a3bc529ccd75c1 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004j-1900000000-5e45f45bce1558c453d6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ik9-9600000000-7f86fd4b1eb78a371954 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0bt9-9100000000-79a8dc902fc1ec97f027 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0feu-8900000000-3c35c8dfcb45f0376fc8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0pbi-9400000000-41db23140c375b3aa0a5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4r-9100000000-cec85f104bcd3341f5e5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00e9-9600000000-38a569c25bb4c854408f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9100000000-7e7f7b55d2df9ddfb991 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ab9-9000000000-d50df044541f569e4776 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dl-9800000000-b7c5e209a4cceb7f6fcd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0r6u-9200000000-89d224c3ada55221e402 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-08fr-9000000000-ad7b84a4ea9ef09d3f08 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|