| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:32:33 UTC |
|---|
| Update Date | 2020-04-22 15:48:32 UTC |
|---|
| BMDB ID | BMDB0012115 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
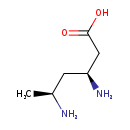
| Common Name | (3S,5S)-3,5-Diaminohexanoate |
|---|
| Description | (3S,5S)-3,5-Diaminohexanoate, also known as (3S,5S)-3,5-diaminocaproic acid or L-erythro-3,5-diaminohexanoate, belongs to the class of organic compounds known as beta amino acids and derivatives. These are amino acids having a (-NH2) group attached to the beta carbon atom (3S,5S)-3,5-Diaminohexanoate exists in all living organisms, ranging from bacteria to humans. Based on a literature review very few articles have been published on (3S,5S)-3,5-Diaminohexanoate. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3S,5S)-3,5-Diaminocaproic acid | ChEBI | | L-Erythro-3,5-diaminohexanoic acid | ChEBI | | L-Erythro-3,5-diaminohexanoate | Kegg | | (3S,5S)-3,5-Diaminocaproate | Kegg | | L-Erythro-3,5-diaminocaproate | Kegg | | L-Erythro-3,5-diaminocaproic acid | Generator | | (3S,5S)-3,5-Diaminohexanoic acid | Generator | | 3,5-diamino-Hexanoate | HMDB | | 3,5-diamino-Hexanoic acid | HMDB | | 3,5-Diaminohexanoate | HMDB | | 3,5-Diaminohexanoic acid | HMDB | | (3S,5S)-3,5-Diaminohexanoate | Generator | | 3,5-Diaminohexanoate dihydrochloride | MeSH, HMDB |
|
|---|
| Chemical Formula | C6H14N2O2 |
|---|
| Average Molecular Weight | 146.1876 |
|---|
| Monoisotopic Molecular Weight | 146.105527702 |
|---|
| IUPAC Name | (3S,5S)-3,5-diaminohexanoic acid |
|---|
| Traditional Name | L-erythro-3,5-diaminohexanoate |
|---|
| CAS Registry Number | 17027-83-7 |
|---|
| SMILES | C[C@H](N)C[C@H](N)CC(O)=O |
|---|
| InChI Identifier | InChI=1S/C6H14N2O2/c1-4(7)2-5(8)3-6(9)10/h4-5H,2-3,7-8H2,1H3,(H,9,10)/t4-,5-/m0/s1 |
|---|
| InChI Key | NGDLSXMSQYUVSJ-WHFBIAKZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as beta amino acids and derivatives. These are amino acids having a (-NH2) group attached to the beta carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Beta amino acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Beta amino acid or derivatives
- Medium-chain fatty acid
- Amino fatty acid
- Fatty acid
- Fatty acyl
- Amino acid
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organopnictogen compound
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Amine
- Primary aliphatic amine
- Carbonyl group
- Organic oxygen compound
- Organic nitrogen compound
- Organic oxide
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000l-9000000000-fe616d85bb79ec3ff09c | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0006-9500000000-37d2c2ce4f47617362dd | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03gj-0900000000-1ac1f45f87957708533b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0239-8900000000-82cfe4ca274f38ceb15b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-06si-9200000000-cb1115634f2f540cbdc5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-a0d8c37141bc7dc8c935 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ufs-2900000000-ef10441acee32815ad0d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-053u-9300000000-af983005a00ac2fc75ae | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001r-7900000000-93da6a650a515a8a47f5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01q9-9400000000-66b0a498aeff05bec142 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dl-9000000000-4a46b2c4530c64cfddde | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-5900000000-6d6ff3949d0dc471dc59 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004l-8900000000-4a00612c8074ae2c39d1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-86e204bc007aae592616 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|