| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:33:04 UTC |
|---|
| Update Date | 2020-04-22 15:48:41 UTC |
|---|
| BMDB ID | BMDB0012143 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
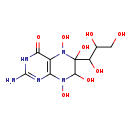
| Common Name | 2-Amino-4-oxo-4-alpha-hydroxy-6-(erythro-1',2',3'-trihydroxypropyl)-5,6,7,8-tetrahydroxypterin |
|---|
| Description | 2-Amino-4-oxo-4-alpha-hydroxy-6-(erythro-1',2',3'-trihydroxypropyl)-5,6,7,8-tetrahydroxypterin, also known as 2-amino-5,6,7,8-tetrahydroxy-6-(1,2,3-trihydroxypropyl)-5,6,7,8-tetrahydro-4(1H)-pteridinone, belongs to the class of organic compounds known as biopterins and derivatives. These are coenzymes containing a 2-amino-pteridine-4-one derivative. They are mainly synthesized in several parts of the body, including the pineal gland. Based on a literature review very few articles have been published on 2-Amino-4-oxo-4-alpha-hydroxy-6-(erythro-1',2',3'-trihydroxypropyl)-5,6,7,8-tetrahydroxypterin. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-6-(erythro-1',2',3'-trihydroxypropyl)-5,6,7,8-tetrahydroxypteridine | ChEBI | | 2-Amino-4-oxo-4-alpha-hydroxy-6-(1',2',3'-trihydroxypropyl)-5,6,7,8-tetrahydroxypterin | ChEBI | | 2-Amino-4-oxo-4-alpha-hydroxy-6-(erythro-1',2',3'-tri-hydroxypropyl)-5,6,7,8-tetrahydroxypterin | ChEBI | | 2-Amino-5,6,7,8-tetrahydroxy-6-(1,2,3-trihydroxypropyl)-5,6,7,8-tetrahydro-4(1H)-pteridinone | ChEBI | | 2-Amino-4-oxo-4-a-hydroxy-6-(1',2',3'-trihydroxypropyl)-5,6,7,8-tetrahydroxypterin | Generator | | 2-Amino-4-oxo-4-α-hydroxy-6-(1',2',3'-trihydroxypropyl)-5,6,7,8-tetrahydroxypterin | Generator | | 2-Amino-4-oxo-4-a-hydroxy-6-(erythro-1',2',3'-tri-hydroxypropyl)-5,6,7,8-tetrahydroxypterin | Generator | | 2-Amino-4-oxo-4-α-hydroxy-6-(erythro-1',2',3'-tri-hydroxypropyl)-5,6,7,8-tetrahydroxypterin | Generator | | 2-Amino-4-oxo-4-a-hydroxy-6-(erythro-1',2',3'-trihydroxypropyl)-5,6,7,8-tetrahydroxypterin | Generator | | 2-Amino-4-oxo-4-α-hydroxy-6-(erythro-1',2',3'-trihydroxypropyl)-5,6,7,8-tetrahydroxypterin | Generator |
|
|---|
| Chemical Formula | C9H15N5O8 |
|---|
| Average Molecular Weight | 321.2441 |
|---|
| Monoisotopic Molecular Weight | 321.092062481 |
|---|
| IUPAC Name | 2-amino-5,6,7,8-tetrahydroxy-6-(1,2,3-trihydroxypropyl)-3,4,5,6,7,8-hexahydropteridin-4-one |
|---|
| Traditional Name | 2-amino-5,6,7,8-tetrahydroxy-6-(1,2,3-trihydroxypropyl)-3,7-dihydropteridin-4-one |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | NC1=NC2=C(N(O)C(O)(C(O)C(O)CO)C(O)N2O)C(=O)N1 |
|---|
| InChI Identifier | InChI=1S/C9H15N5O8/c10-8-11-5-3(6(18)12-8)14(22)9(20,7(19)13(5)21)4(17)2(16)1-15/h2,4,7,15-17,19-22H,1H2,(H3,10,11,12,18) |
|---|
| InChI Key | XWORXFNMQCQKKK-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as biopterins and derivatives. These are coenzymes containing a 2-amino-pteridine-4-one derivative. They are mainly synthesized in several parts of the body, including the pineal gland. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pteridines and derivatives |
|---|
| Sub Class | Pterins and derivatives |
|---|
| Direct Parent | Biopterins and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Biopterin
- Aminopyrimidine
- Pyrimidone
- Pyrimidine
- Heteroaromatic compound
- Vinylogous amide
- Secondary alcohol
- Alkanolamine
- Azacycle
- N-organohydroxylamine
- Polyol
- Organic oxide
- Alcohol
- Primary amine
- Primary alcohol
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Amine
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0w4i-9684000000-6b7e25ba505d22004176 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (5 TMS) - 70eV, Positive | splash10-0002-1142907000-fc2381674ac07bfa292f | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0009000000-5daadb149ed392e168a4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-9024000000-fd1515d6fdeed0c37ace | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-9010000000-1dae7e98b316d4b4e919 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0bu0-1091000000-036d00dad86276a9524b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-9420000000-45f04098e626baba4b83 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0abi-9800000000-ae27aa2d49bde6cf0702 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0009000000-bb5a068c8c836021e5af | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-3309000000-336f1484703b07f7a223 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dl-2890000000-8abd2b1566c71f6497ee | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0090000000-053dfb1e66e9a1505d5e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03dl-0290000000-97615aaa57b6712fd068 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03du-6590000000-43cfe1e05991792e18ef | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|