| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2018-07-17 17:23:54 UTC |
|---|
| Update Date | 2020-04-22 15:51:47 UTC |
|---|
| BMDB ID | BMDB0062263 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Tryptophyl-Arginine |
|---|
| Description | Tryptophyl-arginine, also known as W-R dipeptide or TRP-arg, belongs to the class of organic compounds known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. Tryptophyl-arginine is possibly soluble (in water) and a very strong basic compound (based on its pKa). |
|---|
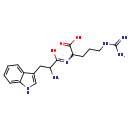
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| L-Tryptophyl-L-arginine | HMDB | | TRP-Arg | HMDB | | Tryptophan arginine dipeptide | HMDB | | Tryptophan-arginine dipeptide | HMDB | | Tryptophylarginine | HMDB | | W-R Dipeptide | HMDB | | WR Dipeptide | HMDB | | 2-{[2-amino-1-hydroxy-3-(1H-indol-3-yl)propylidene]amino}-5-carbamimidamidopentanoate | HMDB |
|
|---|
| Chemical Formula | C17H24N6O3 |
|---|
| Average Molecular Weight | 360.4109 |
|---|
| Monoisotopic Molecular Weight | 360.190988664 |
|---|
| IUPAC Name | 2-{[2-amino-1-hydroxy-3-(1H-indol-3-yl)propylidene]amino}-5-carbamimidamidopentanoic acid |
|---|
| Traditional Name | 2-{[2-amino-1-hydroxy-3-(1H-indol-3-yl)propylidene]amino}-5-carbamimidamidopentanoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | NC(CC1=CNC2=CC=CC=C12)C(O)=NC(CCCNC(N)=N)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C17H24N6O3/c18-12(8-10-9-22-13-5-2-1-4-11(10)13)15(24)23-14(16(25)26)6-3-7-21-17(19)20/h1-2,4-5,9,12,14,22H,3,6-8,18H2,(H,23,24)(H,25,26)(H4,19,20,21) |
|---|
| InChI Key | LCPVBXOHXMBLFW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Dipeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-dipeptide
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid amide
- Triptan
- Alpha-amino acid or derivatives
- 3-alkylindole
- Indole
- Indole or derivatives
- Aralkylamine
- Fatty amide
- Substituted pyrrole
- Benzenoid
- Fatty acyl
- Pyrrole
- Heteroaromatic compound
- Amino acid or derivatives
- Carboxamide group
- Guanidine
- Amino acid
- Secondary carboxylic acid amide
- Carboximidamide
- Azacycle
- Organoheterocyclic compound
- Carboxylic acid
- Organic 1,3-dipolar compound
- Monocarboxylic acid or derivatives
- Propargyl-type 1,3-dipolar organic compound
- Organic oxide
- Organopnictogen compound
- Amine
- Organic nitrogen compound
- Primary aliphatic amine
- Carbonyl group
- Organic oxygen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Organooxygen compound
- Primary amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-053f-9711000000-299d9a57ea6092e0e50c | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0a4i-3910000000-a3ce8c66b41de0de6b3a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-08fu-0609000000-014cd4cb60306e79d1d3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6u-1901000000-62438ab4736b313f2d47 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-06sl-3900000000-42f3a50ff58c76cb444b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0aor-1019000000-8e94df357b4a5c981191 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0aor-7679000000-68a947d89cf629c97053 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9300000000-741ba16fa1c9a4811a27 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0209000000-9bdbdd314110400fdef4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0913000000-56ec595b2f26cc35f8bb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-1910000000-9029089ef577cbc958bf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0119000000-f144f60bef59a7c944a4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-5988000000-2bc2c3e46ad20b6b45b2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9400000000-527014684221ebf20aa6 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|
| General References | - Pan L, Yu J, Mi Z, Mo L, Jin H, Yao C, Ren D, Menghe B: A Metabolomics Approach Uncovers Differences between Traditional and Commercial Dairy Products in Buryatia (Russian Federation). Molecules. 2018 Mar 22;23(4). pii: molecules23040735. doi: 10.3390/molecules23040735. [PubMed:29565828 ]
|
|---|